 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
Therapeutic antibodies offer valuable tools in the medical field for treating a variety of disorders. They have emerged as one of the dominant therapeutic modalities, with over 50 approved products and over 500 monoclonal antibody (mAb)-based therapies in clinical development. Antibodies are natural macromolecules that have a high affinity and specificity for binding to a wide range of antigenic targets by utilizing unique pharmacokinetic (PK) and pharmacodynamic (PD) properties. The limitations of antibodies result mainly from their large size and poor penetration in solid tissues.
About 30 years ago, functional heavy chain antibodies were accidentally discovered in the serum of camels (dromedaries, camels, llamas, guanacos and vicuñas). The variable domain of this heavy-chain antibody includes complete antigen binding potential and strong affinity with homologous antigens, so it is the smallest naturally occurring complete antigen binding fragment. Based on heavy-chain antibodies, ABLYNX (acquired by Sanofi in 2018) has developed antibodies that contain only VHH fragments. Because of its tiny nanoscale size, ABLYNX proposed the concept of "Nanobody".
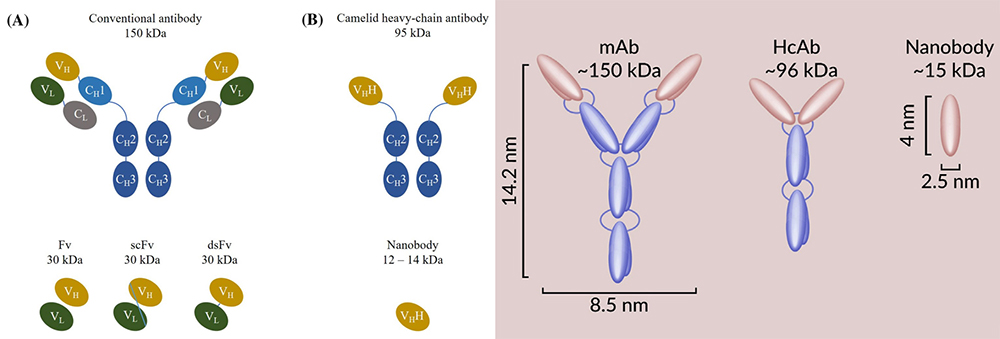
Different structure of antibodies: mAb, HcAb (heavy chain antibody), Nanobody
Nanobody is a novel, unique and complete antigen-binding fragment with simple structure, small molecular weight (about 15kDa, which is 10 times smaller than monoclonal antibodies), good physical and chemical stability, good water solubility, strong tissue permeability and can penetrate into blood-brain barrier. Nanobody can be efficiently produced by recombinant microorganisms, with low cost and easy to assemble or merge into more complex multi-energy structures. Combined the advantages of traditional antibodies and small molecular drugs, Nanobody almost perfectly overcomes the defects of traditional antibodies with long development cycles, low stability, and harsh storage conditions. It has gradually become an emerging force in a new generation of therapeutic biomedicine and clinical diagnostic reagents.
A considerable shortcoming of Nanobody is that they are quickly cleared in the blood, which leads to a short half-life of the drug. In view of this problem, the long-acting platform technology of protein drugs can be used that prolongs action duration of these biologics. It mainly includes two strategies:
1) Increase the hydraulic radius of protein drugs and reduce the glomerular filtration rate.
2) Use of binding balance, release of drug and plasma protein and extend the half-life.
Based on the above two strategies, latest technologies include polyethylene glycol (PEG) modification, HSA(Human Serum Albumin) fusion, Fc fusion, etc. PEG modification is to increase the hydrodynamic radius and extend the half-life of the drug by introducing hydrophilic PEG chain molecules, albumin fusion or Fc fusion is to extend the half-life of the drug by means of a pH-dependent FcRn recirculation pathway (click to view the mechanism of FcRn-mediated half-life extension). Additionally, some emerging technologies are gradually moving from behind the scenes to front of stage, such as ABD fusion, a polypeptide segment that binds to albumin, XTEN fusion, which increases molecular weight by fusing inert proteins, and CTP fusion, which introduces glycosylation modification sites.
HSA(Human Serum Albumin) is a therapeutic protein that is mainly used for drug delivery and disease diagnosis. HSA-fusion Nanobody can increase the half-life of the drug and improve its efficacy.
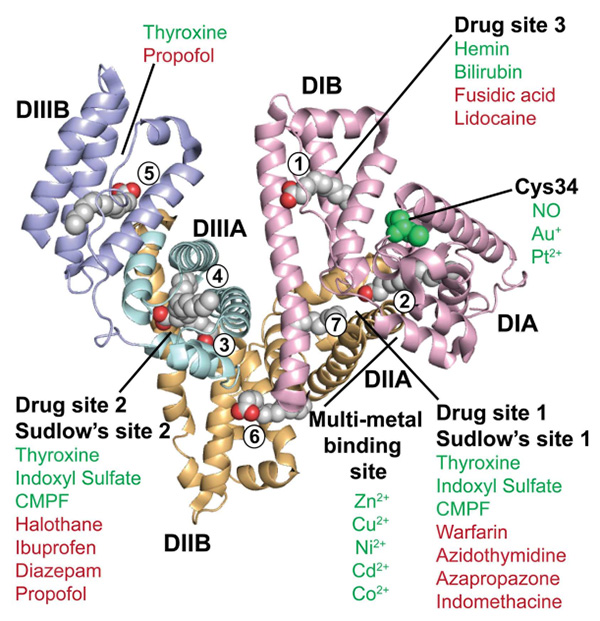
Crystalline structure of HSA
The X-ray crystal structure of HSA shows that it is a heart-shaped molecule containing 585 amino acids. It is mainly composed of an α-helical structure and lacks β-folding. It is combined into three homologous domains DI, DII and DIII, each of which is divided into A and B subdomains (DIA, DIB, DIIA, DIIB, DIIIA and DIIIB). These domains are connected by long and flexible rings. HSA is the main protein in plasma, with a concentration of about 45 mg/ml (0.6 mm) and a half-life of 20 days. Ablynx's exclusive half-life extension technology combines Nanobody with HSA to increase the molecular weight of specific antibodies. They have used the recirculation effect of FcRn to extend the half-life from few hours to more than 3 weeks.
Meanwhile, in addition to its short half-life, Nanobody does not possess Fc-mediated effector functions due to the lack of Fc fragments of antibodies, such as for anti-tumor therapy. It also lacks the antibody-dependent cell-mediated cytotoxicity (ADCC) and serum stability brought about by Fc. Natural killer (NK) cells are the vital immune cells that mediate the action of ADCC, and the Fc receptor FcyRIIIa (CD16A) on the cell surface plays a key role in recognizing and binding Fc.
Nanobody can be easily equipped with Fc regions (hinge, CH2, and CH3 domains) in the form of recombinant humanized heavy-chain antibodies to achieve effector functions including Fc-mediated and increase blood residence time, but it will inevitably reduce its diffusion advantage. Fc-fusion fragments is more inclined to choose IgG1 Fc, which is related to the affinity binding properties of IgG1 to the Fc receptor proteins.
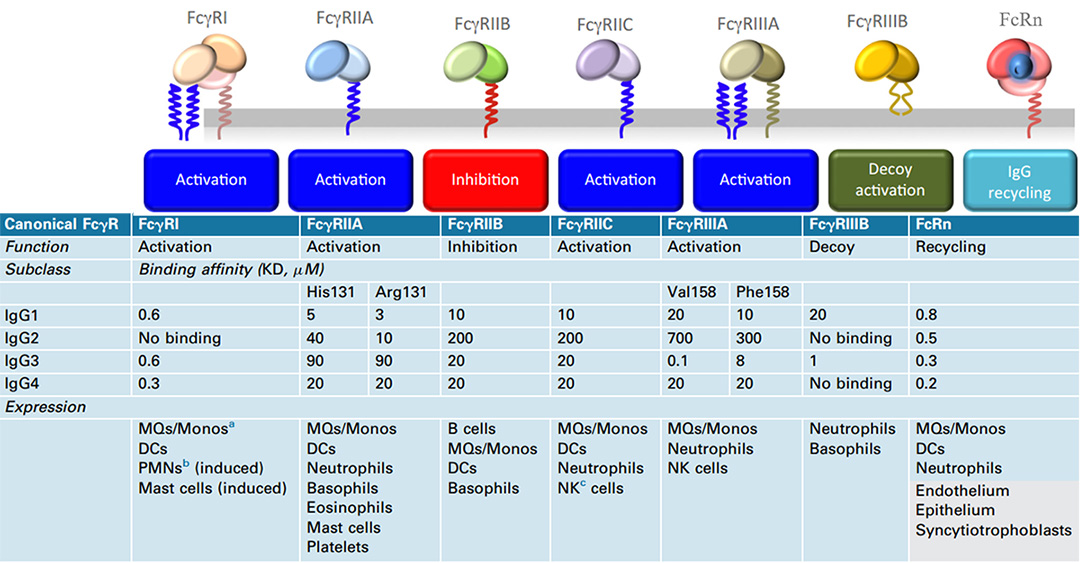
Human canonical FcγR, functions, binding affinities to various IgG subclasses, and expression in cells
As shown in the table below, the Fc-fusion drugs that have been approved by the FDA contain the human IgG1 Fc domain, and their main purpose is to extend the serum half-life of the active part.
Marketed Fc-fusion drugs
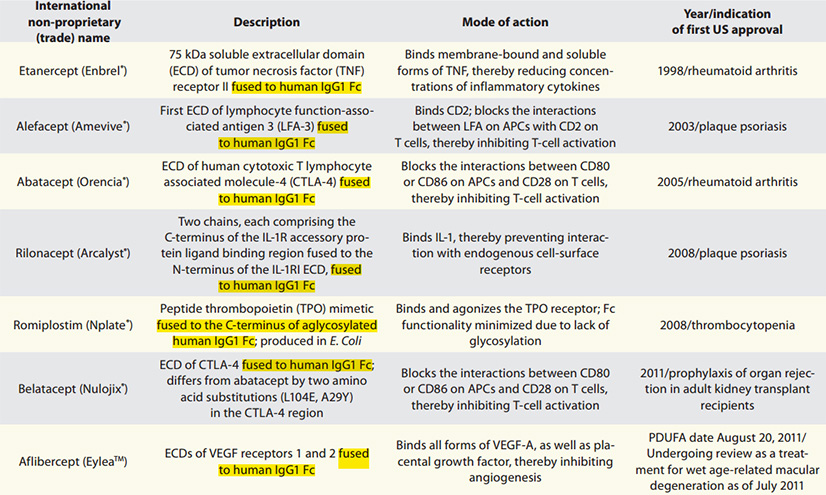
In addition to increasing the half-life, Fc-fusion also improves the stability and solubility of the drug. It is beneficial to the production process, because the fusion expression of Fc is conducive to the affinity purification of protein A, thereby simplifying the downstream purification process of protein drugs. Therefore, Fc-fusion proteins have achieved considerable success in terms of the number of approved products, applications in different disease fields, and global value.
Antibodies are the main macromolecules used in targeted therapy, which can significantly improve the clinical care and life quality of cancer patients. However, the limitations of antibodies in terms of size, incomplete tumor penetration, and possible immunogenicity have led to the development of a new generation of small drugs.
Nanobody not only provides new possibilities for the treatment of cancer, but also provides new therapeutic possibilities for various human diseases at the subcellular level, which will revolutionize the field of biomedicine. Due to its small size and good tissue permeability, it may become an ideal diagnostic and therapeutic tool. However, due to the lack of functional Fc, the half-life is very short and the clearance rate in the kidneys is very fast.
HSA and Fc fusion technologies confer more antibody-like properties to proteins and peptides of therapeutic value. Currently, Nanobodies are widely used in clinical development and treatment fields including inflammation, infectious diseases, and hematology. For indications of chronic diseases, Nanobody prolonged exposure is essential. Its half-life-prolonging structure can be produced by fusing HSA or Fc. In acute indications, non-half-life-prolonging forms may show stronger kinetic advantages.
Furthermore, introducing anti-HSA or anti-FcR to Nanobody, mediating the binding of the drug to HSA can also effectively increase the half-life of the drug. A series of bispecific Nanobodies based on anti-tumor antigen, anti-CD16 and anti-HSA Nanobodies have been reported. There is no doubt that reasonable drug design can make Nanobody fully demonstrate its potential.
To assist in the development of Nanobody drug, ACROBiosystems is providing you following series of products:
Hot target proteins, suitable for immunization, screening, process method development and optimization
HSA and IgG Fc with homogeneous structure, verified activity, and strict standards to meet the development of HSA-fusion and Fc-fusion products, especially in the process of functional verification and method development for the requirements of isotype control. During the development of anti-HSA Nanobody drugs, HSA can be used as bihding ligand.
Full range of high-quality Fc receptor proteins: FcRn, FcγRI/CD64, FcγRIIA/CD32a, FcγIIB/CD32B, FcγRIIIa/CD16A, FcγRIIIa/CD16B, suitable for verifing the half-life and binding affinity to Fc receptor proteins of Nanobody drug fused with Fc fragments.
| Molecule | Cat. No. | Species | Product Description |
|---|---|---|---|
| Serum Albumin | HSA-H5220 | Human | Human Serum Albumin Protein, His Tag (HPLC verified) |
| Serum Albumin | HSA-H82E3 | Human | Biotinylated Human Serum Albumin Protein, His,Avitag™ |
| Serum Albumin | HSA-H522a | Human | Human Serum Albumin Protein, His Tag, low endotoxin |
| Serum Albumin | MSA-M52H8 | Mouse | Mouse Serum Albumin Protein, His Tag |
| Serum Albumin | MSA-M82E4 | Mouse | Biotinylated Mouse Serum Albumin Protein, His,Avitag™ (MALS verified) |
| Serum Albumin | CSA-C52H4 | Cynomolgus | Cynomolgus Serum Albumin Protein, His Tag |
| Serum Albumin | RSA-R52H6 | Rabbit | Rabbit Serum Albumin Protein, His Tag |
 The SA sequence is a complete natural sequence with only the signal peptide and propeptide removed
The SA sequence is a complete natural sequence with only the signal peptide and propeptide removed
 Various species: Human, Mouse, Cynomolgus, Rabbit
Various species: Human, Mouse, Cynomolgus, Rabbit
 High purity: more than 95% as verified by SDS-PAGE and more than 90% as verified by SEC-MALS
High purity: more than 95% as verified by SDS-PAGE and more than 90% as verified by SEC-MALS
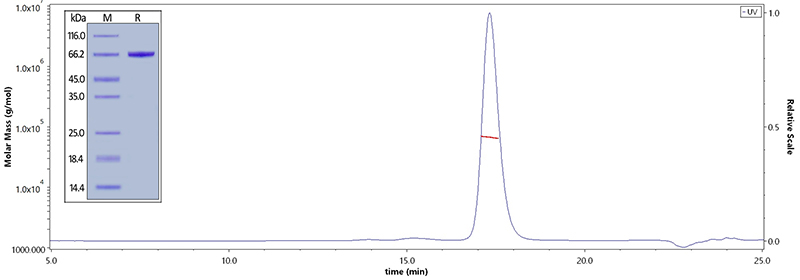
Human Serum Albumin, His Tag (Cat. No. HSA-H5220) on SDS-PAGE under reducing (R) condition. The purity of the protein is greater than 95%. The purity of Human Serum Albumin, His Tag (Cat. No. HSA-H5220) is more than 90% and the molecular weight of this protein is around 60-75 kDa verified by SEC-MALS.
 Bioactivity verified by SPR
Bioactivity verified by SPR
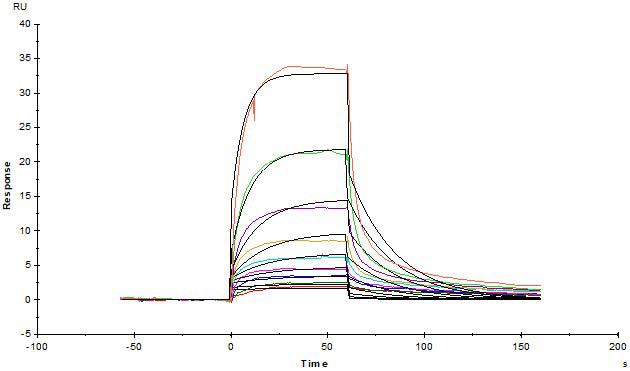
Human Serum Albumin Protein, His Tag (Cat. No. HSA-H5220) immobilized on CM4 Chip can bind Human FCGRT&B2M Heterodimer Protein, His Tag (Cat. No. FCN-H52W7) with an affinity constant of 0.381 μM as determined in a SPR assay (Biacore T200).
>>> Click here to view serum albumin (SA) products and verification data
 IgG Fc: structure and application
IgG Fc: structure and application
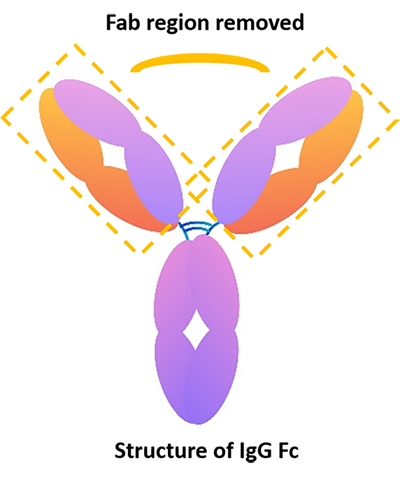
 What can IgG Fc be used for?
What can IgG Fc be used for?
As an isotype control of IgG subtype antibody, IgG Fc is used for antibody screening and functional verification, etc.
 How does IgG Fc achieve the effect of isotype control?
How does IgG Fc achieve the effect of isotype control?
Recombinant protein IgG Fc contains constant region sequences including hinge region, CH2, CH3, but does not contain sequences of Fab region. So, it can be used as a control to clarify the specific role of your antibody in recognizing the target antigen. For example, if the result of the IgG Fc isotype control group is negative, the result of the experimental group truly reflects the effect of the variable region of the antibody.
| Molecule | Cat. No. | Species | Product Description |
|---|---|---|---|
| IgG1 Fc | FCC-H5214 | Human | Human IgG1 Fc Protein, Tag Free (MALS verified) |
| IgG1 Fc | IG1-H8213 | Human | Biotinylated Human IgG1 Fc protein, Avitag™ (MALS verified) |
| IgG1 Fc | IG1-H5225 | Human | Human IgG1 Fc Protein, His Tag (MALS verified) |
| IgG1 Fc | IG1-H52C9 | Human | Human IgG1 Fc Protein, Flag Tag (MALS verified) |
| IgG1 Fc | IG1-H52G6 | Human | Human IgG1 Fc Protein, gD Tag (MALS verified) |
| IgG2 Fc | IG2-H5206 | Human | Human IgG2 Fc Protein, Tag Free (SPR verified) |
| IgG3 Fc | IG3-H5200 | Human | Human IgG3 Fc Protein, Tag Free (SPR verified) |
| IgG4 Fc | IG4-H5205 | Human | Human IgG4 Fc Protein, Tag Free (MALS & SPR verified) |
| IgG1 Fc | IG1-M5208 | Mouse | Mouse IgG1 Fc Protein, Tag Free (HPLC verified) |
| IgG1 Fc | IG1-M8211 | Mouse | Biotinylated Mouse IgG1 Fc protein, Avitag™ |
| IgG2a Fc | IGA-M5207 | Mouse | Mouse IgG2a Fc Protein, Tag Free (MALS verified) |
| IgG2a Fc | IGA-M8210 | Mouse | Biotinylated Mouse IgG2a Fc Protein, Avitag™ (MALS verified) |
| IgG2b Fc | IGB-M5203 | Mouse | Mouse IgG2b Fc Protein, Tag Free (MALS verified) |
| IgG2b Fc | IGB-L5204 | Llama | Llama IgG2b Fc Protein, Tag Free (MALS verified) |
| IgG Fc | IGG-R5203 | Rabbit | Rabbit IgG Fc Protein, Tag Free (MALS verified) |
 More product options: Human IgG1 Fc,IgG2 Fc,IgG3 Fc,IgG4 Fc,Mouse IgG1 Fc,IgG2a Fc,IgG2b Fc,Llama IgG2b Fc,Rabbit IgG Fc
More product options: Human IgG1 Fc,IgG2 Fc,IgG3 Fc,IgG4 Fc,Mouse IgG1 Fc,IgG2a Fc,IgG2b Fc,Llama IgG2b Fc,Rabbit IgG Fc
 Expressed by HEK293 cell which realize post-translational glycosylation and other modifications and correct protein folding
Expressed by HEK293 cell which realize post-translational glycosylation and other modifications and correct protein folding
 Different tags to meet the needs of different application: Tag Free,AvitagTM,His tag,gD tag,Flag tag,AvitagTM & His tag
Different tags to meet the needs of different application: Tag Free,AvitagTM,His tag,gD tag,Flag tag,AvitagTM & His tag
 High purity: more than 95% as verified by SDS-PAGE and more than 95% as verified by SEC-MALS
High purity: more than 95% as verified by SDS-PAGE and more than 95% as verified by SEC-MALS
 Low endotoxin: Less than 1.0 EU/μg by the LAL method
Low endotoxin: Less than 1.0 EU/μg by the LAL method
 Bioactivity verified by ELISA & SPR
Bioactivity verified by ELISA & SPR
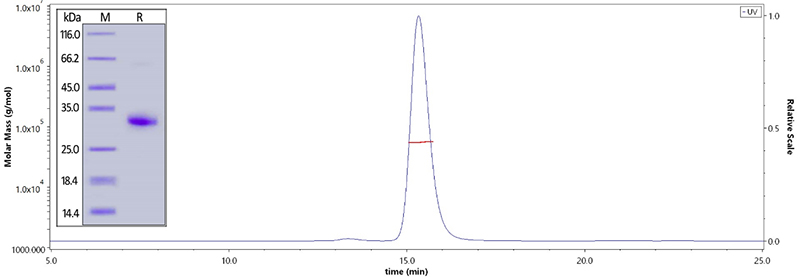
Human IgG Fc, Tag Free (Cat. No. FCC-H5214) on SDS-PAGE under reducing (R) condition. The purity of the protein is greater than 95%. The purity is more than 95% and the molecular weight of this protein is around 51-65 kDa verified by SEC-MALS.
 Bioactivity verified by ELISA&SPR
Bioactivity verified by ELISA&SPR
>>> Click here to view IgG Fc products and verification data
[1] Ivana Jovčevska1, Serge Muyldermans. The therapeutic potential of nanobodies. BioDrugs. 2020, 34: 11–26
[2] Elmira Karami, Mahdi Behdani, et al. Albumin nanoparticles as nanocarriers for drug delivery: Focusing on antibody and nanobody delivery and albumin-based drugs. Journal of Drug Delivery Science and Technology. 2020, 55: 101471
[3] Zhimin Xu , Chuangnan Qiu, et al. A bispecific nanobody targeting the dimerization interface of epidermal growth factor receptor: Evidence for tumor suppressive actions in vitro and in vivo. Biochemical and Biophysical Research Communications. 2021, 548: 78-83
[4] Kine Marita Knudsen Sand, Malin Bern, et al. Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Frontiers in Immunology. 2015, 5, 682
[5] Alain Beck, Janice M. Reichert. Therapeutic Fc-fusion proteins and peptides as successful alternatives to antibodies. Landes Bioscience. 2011, 3(5): 415-416
[6] Liming Liu. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins.Protein Cell. 2018, 9(1): 15–32
[7] Ditza Levin1, Basil Golding, et al. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends in Biotechnology. 2015, 33(1): 27-34
This web search service is supported by Google Inc.
