Background
According to the World Health Organization (WHO), various brain-related diseases including degenerative, functional, and psychiatric disorders, have become a major public health concern. Their impact on social and economic outcomes is even great than cardiovascular diseases and cancers. According to the World Alzheimer Report, in 2015, there were 46,800,000 Alzheimer's patients worldwide, with an additional 9,900,000 new cases globally. This is equivalent to one new case every three seconds. With case numbers expected to is expected to grow to 74,700,000 by 2030 and to 130,000,000 by 2050.
The diagnosis and intervention of major brain-related diseases are at the forefront of brain research. This situation actively inspires to promote the development of the drug industry and new bio-engineering companies. Improving the management of brain-related disease is thus expected to play an important part in improving the health and well-being of modern society.
As a leading manufacturer of recombinant proteins and other critical reagents for support in developing target therapeutics, vaccines, and diagnostics, ACROBiosystems employs an application-oriented development strategy, with a particular focus on product design, quality control, and solution-based support.
Aneuro is a new product line of ACRO that focuses and reflects dedicated efforts in neuroscience research. We aim to promote and facilitate neuroscience research by providing high-quality protein products and valuable new ideas.
Products for Therapeutic Research
Alzheimer′s disease (AD)
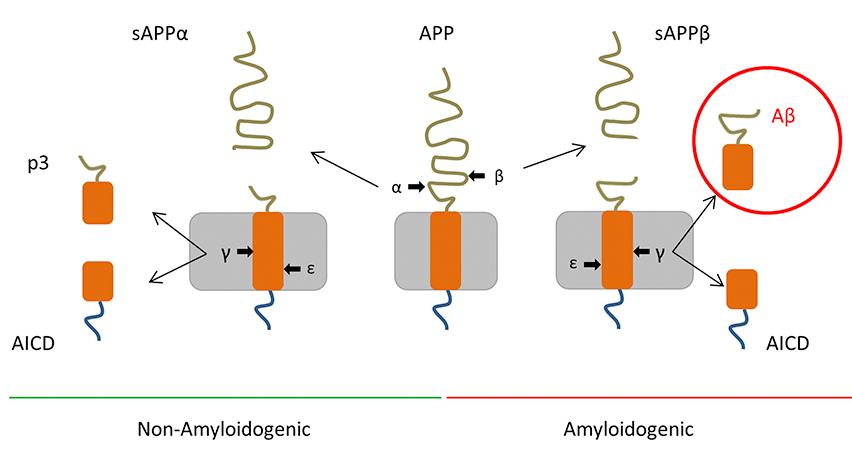
Alzheimer′s disease (AD)is a progressive neurodegenerative disorder that affects the central neuron system. Specific symptoms include impairments in cognitive functions such as memory and changes in behavior. Currently, there is no cure for AD with existing treatments mainly focusing on restoring the cognitive functions and slowing down the disease progression. At present, the exact mechanism of AD pathogenesis is still under debate, with existing hypotheses including the β-amyloid waterfall theory, the Tau protein theory, and the neurovascular hypothesis.
https://www.sciencedirect.com/science/article/pii/S1552526016000790
Schematic presentation of the APP proteolytic processes
Parkinson disease (PD)
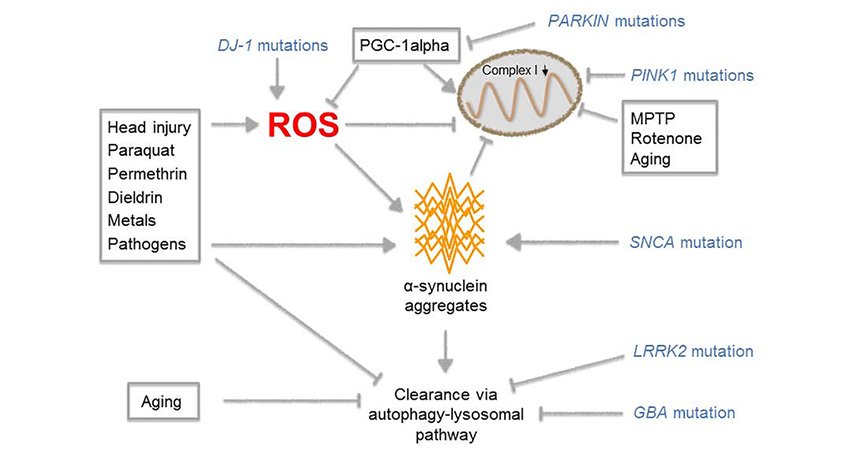
Parkinson’s disease (PD) is a progressive neurodegenerative disease that is most commonly diagnosed in middle-aged and elderly people. The typical clinical symptoms involve bradykinesia, rigidity, tremor, and poor posture at the late stage of the disease. PD brain shows loss of pigment and dopaminergic neurons in the substantia nigra and is associated with the emergence of Lewis bodies and the increase of glial. Consequently, an imbalance between striatum inhibitory transmitter dopamine and excitatory transmitter acetylcholine occurs.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6905381/
Multiple pathways that influence the onset of PD
Huntington disease (HD)
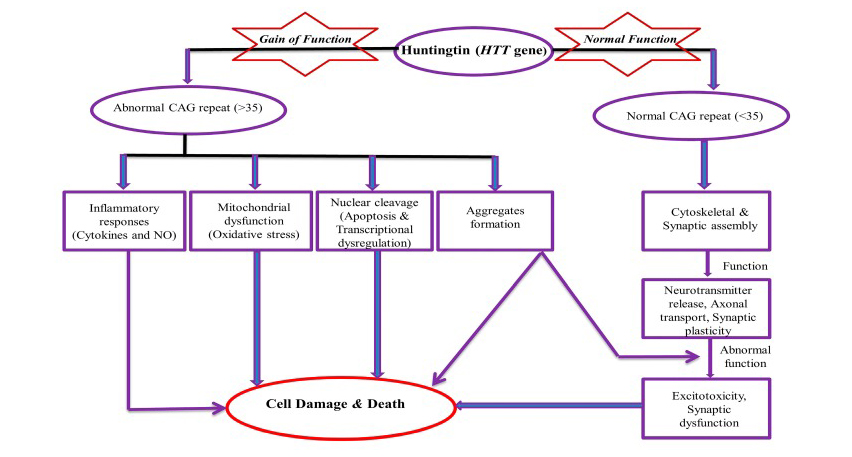
Huntington’s disease (HD), is a rare, neurodegenerative disease that is inherited in an autosomal dominant manner. It causes progressive physiological, psychiatric and cognitive disorders. HD is caused by an abnormal expansion of the CAG trinucleotide repeat on the HTT gene located at chromosome 4.
https://pubmed.ncbi.nlm.nih.gov/31940909/
Mechanism of Toxicity of Huntingtin (HTT) gene
Amyotrophic lateral sclerosis (ALS)
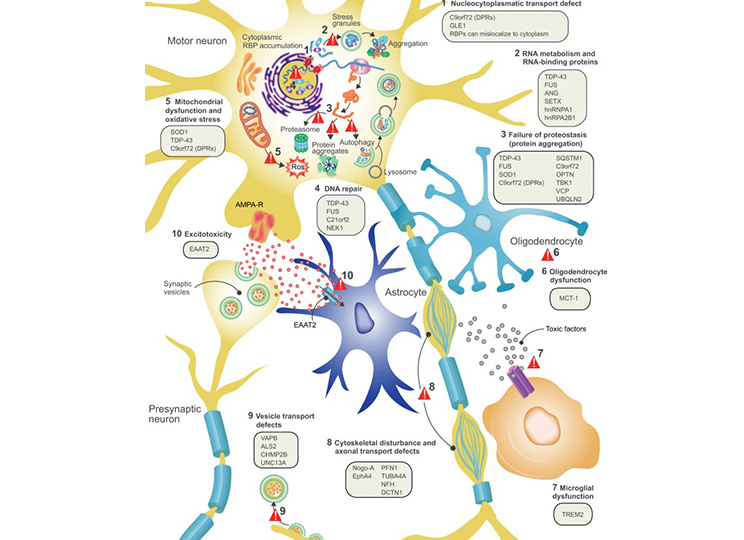
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the progressive degeneration of both the upper and lower motor neurons. The disease causes gradual weakening and atrophy of the limbs, trunk, chest, and abdominal muscles. It affects movement, communication, swallowing, and breathing eventually leading to death. Mutations in the SOD1 gene have been found to be associated with familial ALS and is thought to be a leading genetic factor in the progression of the disease.
https://pubmed.ncbi.nlm.nih.gov/28468939/
ALS disease pathology and proposed disease mechanisms
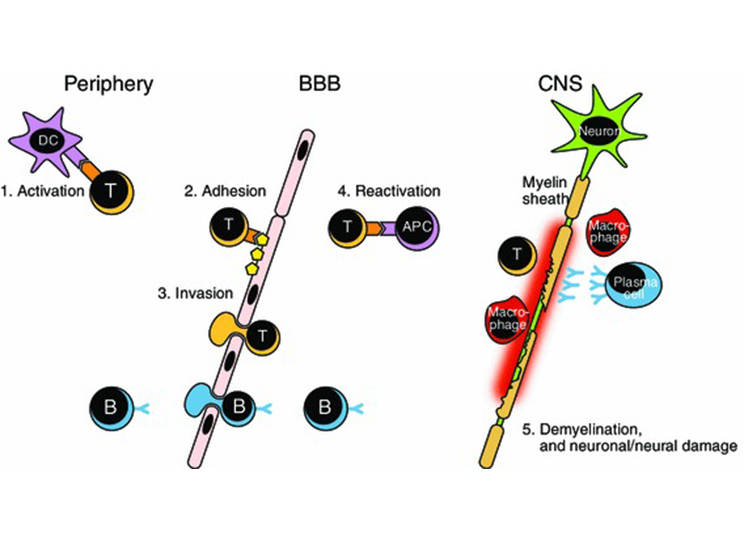
Multiple sclerosis (MS)
Multiple sclerosis (MS) is an autoimmune-mediated neurodegenerative disease characterized by inflammatory demyelination of nerves in the central nervous system. Various studies have emphasized the role of B cells and the autoantibodies they produce in the pathogenesis of MS. Pharmaceutical companies such as Roche and Novartis have developed a variety of CD20 antibody drugs specifically targeting B cells to treat multiple sclerosis.
https://pubmed.ncbi.nlm.nih.gov/24740824/
The roles of immune cells in multiple sclerosis pathogenesis
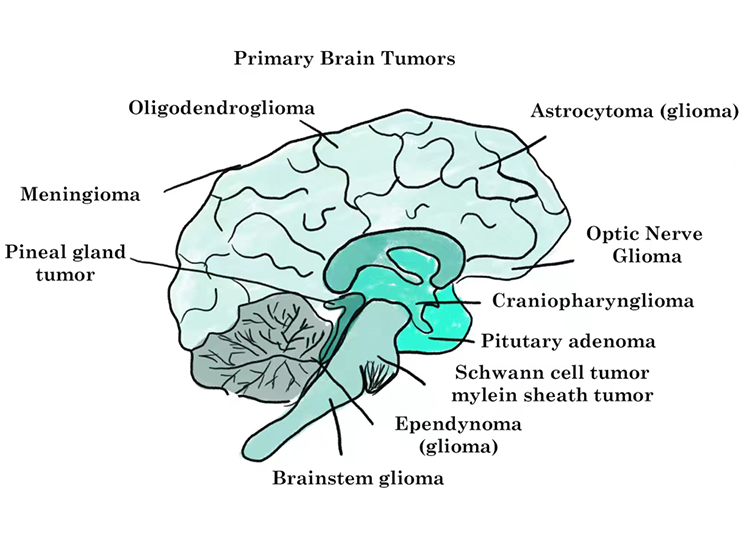
Neurosystem-related Neoplasms
Brain tumors can be subdivided into different tumor types, commonly including gliomas, meningiomas, gliomas, medulloblastomas, etc.
The treatment for a brain tumor will depend on many things, including the type, size and location of the tumor, as well as symptoms, general health and treatment preferences. Targeted drug therapy is one of the main treatment options for a brain tumor.
https://brainmadesimple.com/brain-tumor-cancer/
Different categories of brain tumors
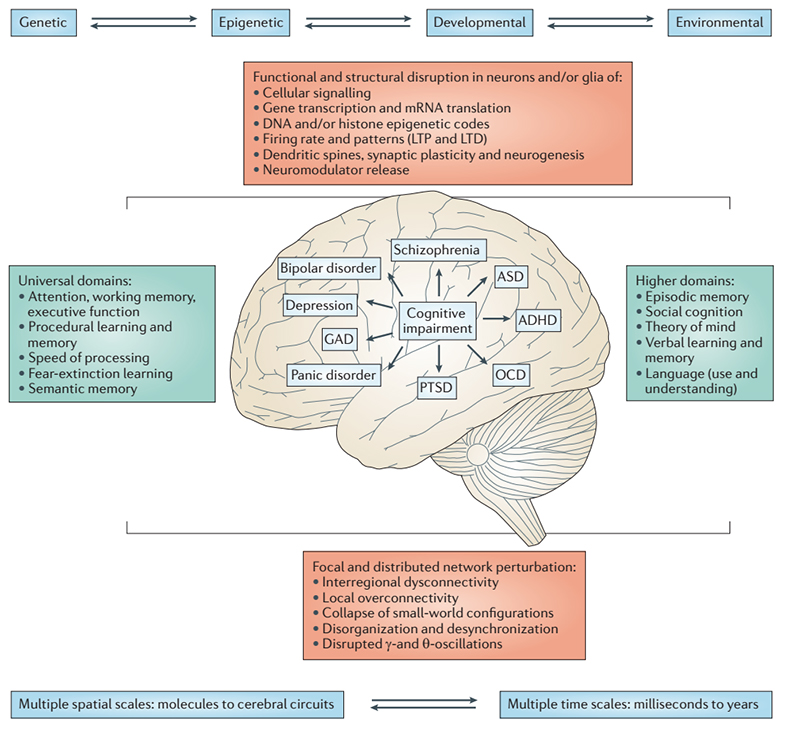
Functional & Psychiatric Disorders
Functional neurological disorders include epilepsy, attention deficit and autism. These disorders have complex causes and are influenced by a variety of genetic and environmental factors. Neuropsychiatric diseases include schizophrenia, bipolar disorder, depression, anxiety, etc. This kind of disease used to be considered as a simple psychological disease, but more and more studies have proved that it is a kind of complex brain and nervous system diseases and found the organic and molecular pathological characteristics. For example, Neurogranin (Ng), a brain-specific protein kinase C substrate, has been associated with abnormal expression of Ng in a variety of nervous system-induced cognitive impairments such as AD, stroke, and schizophrenia.
https://doi.org/10.1038/nrd3628
Cognitive summary of functional neurological disorders
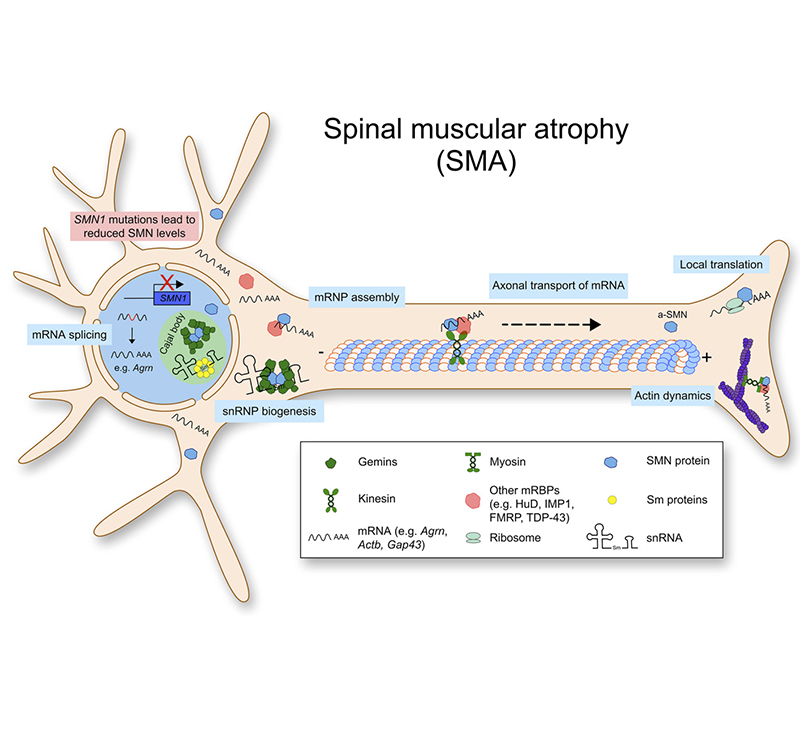
Spinal Muscular Atrophy (SMA)
SMA is a progressive inferior motor neuron damage disease with high fatal, disabling, and rare characteristics. It was included in the first Batch of Rare Diseases List in 2018. The study found that mutations in the SMN1 gene encoding the motor neuron survival protein (SMN), resulting in insufficient production of SMN protein, are known to be the main cause of spinal muscular atrophy. SMN1 mutations include exon 7 and/or 8 deletion (95% +) and point mutations. There are currently several approved drugs targeting the SMN1 gene, and several are in clinical development. Treatments approved by the FDA and EMA are designed to increase the expression of SMN protein, either by antisense oligonucleotides or small molecule drugs to inhibit the exon 7 jump of SMN2, or by viral gene transfer to introduce a complete additional cDNA copy of SMN1.
https://doi.org/10.3389/fmolb.2019.00161
Pathogenesis of SMA
If you have any needs or more development suggestions, please feel free to contact us
Leave a message
Products for Diagnostic Research
At present, neuro-related diseases represented by neurodegenerative diseases are still in the situation of low diagnosis rate, high misdiagnosis rate, and low treatment rate. Therefore, early diagnosis is particularly important for timely intervention and treatment. Based on diagnosis and treatment guidelines, Aneuro provides a series of high-quality proteins covering comprehensive diagnostic indicators in order to facilitate the in vitro diagnostic research of neurological diseases.
| Molecule | Cat. No. | Species | Product Description |
|---|
| APP/A beta | APP-H51H7 | Human | Human APP / Abeta40 Protein, His Tag |
| APP-H52H5 | Human | Human APP / SAPPbeta Protein, His Tag |
| APP-M52H3 | Mouse | Mouse APP / N-APP Protein, His Tag |
| Alpha-synuclein | ALN-H52H8 | Human | Human Alpha-Synuclein Protein, His Tag |
| ALN-H82H8 | Human | Biotinylated Human Alpha-Synuclein Protein, His,Avitag™ |
| ALN-H5253 | Human | Human Alpha-Synuclein Protein, Fc Tag (MALS verified) |
| ALN-H5116 | Human | Human Alpha-Synuclein (A53T) Protein, Tag Free (MALS verified) |
| ALN-H5117 | Human | Human Alpha-Synuclein (E46K) Protein, Tag Free |
| ALN-H5214 | Human | Human Alpha-Synuclein Protein, Tag Free (MALS verified) |
| ALN-H51H5 | Human | Human Alpha-Synuclein (A53T) Protein, His Tag |
| ALN-M52H6 | Mouse | Mouse Alpha-Synuclein Protein, His Tag (MALS verified) |
| ALN-C52H5 | Cynomolgus | Cynomolgus Alpha-Synuclein Protein, His Tag (MALS verified) |
| GFAP | GFP-H5143 | Human | Human GFAP Protein, His Tag |
| GFP-M5148 | Mouse | Mouse GFAP Protein, His Tag |
| GAD1 | GA1-H5543 | Human | Human GAD1 / GAD67 Protein, His Tag |
| GAD2 | GA2-H55H3 | Human | Human GAD2 / GAD65 Protein, His Tag |
| MOG | MOG-H52H3 | Human | Human MOG Protein, His Tag |
| Neuron-specific Enolase(NSE) | NSE-H5144 | Human | Human NSE Protein, His Tag |
| NSE-M5147 | Mouse | Mouse NSE Protein, His Tag |
| Neurofilament Light (NFL) | NFL-H5143 | Human | Human NFL Protein, His Tag |
| Neurofilament Heavy (NFH) | NFH-H5544 | Human | Human NFH Protein, His Tag |
| S100B | S1B-H5143 | Human | Human S100B Protein, His Tag |
| NPTX2 | NP2-H52H6 | Human | Human NPTX2 Protein, His Tag (MALS verified) |
| Tau | TAU-H51H3 | Human | Human Tau-441 / 2N4R Protein, His Tag |
| TAU-H51H5 | Human | Human Tau-441 / 2N4R (273-380) Protein, His Tag (MALS verified) |
| TAU-H51H4 | Human | Human Tau-441 / 2N4R (241-380) Protein, His Tag (MALS verified) |
| TAU-H5143 | Human | Human Tau-410 / 2N3R Protein, His Tag |
| TAU-H5144 | Human | Human Tau-441 / 2N4R (P301L) Protein, His Tag |
| TAU-H5145 | Human | Human Tau-441 / 2N4R (P301S) Protein, His Tag |
| TAU-H5117 | Human | Human Tau-441 / 2N4R Protein, Tag Free (MALS verified) |
| TAU-H5115 | Human | Human Tau-441 / 2N4R Pre-formed Fibrils Protein, Tag Free |
References
1. Myasnikov, A., H. Zhu, P. Hixson, B. Xie, K. Yu, A. Pitre, J. Peng and J. Sun (2021). "Structural analysis of the full-length human LRRK2." Cell 184(13): 3519-3527 e3510.
2. Knopman, D. S., H. Amieva, R. C. Petersen, G. Chetelat, D. M. Holtzman, B. T. Hyman, R. A. Nixon and D. T. Jones (2021). "Alzheimer disease." Nat Rev Dis Primers 7(1): 33.
3. Bloem, B. R., M. S. Okun and C. Klein (2021). "Parkinson's disease." Lancet 397(10291): 2284-2303.
4. Tabrizi, S. J., M. D. Flower, C. A. Ross and E. J. Wild (2020). "Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities." Nat Rev Neurol 16(10): 529-546.
5. Reich, D. S., C. F. Lucchinetti and P. A. Calabresi (2018). "Multiple Sclerosis." N Engl J Med 378(2): 169-180.
6. Pan, X., Z. Li, Q. Zhou, H. Shen, K. Wu, X. Huang, J. Chen, J. Zhang, X. Zhu, J. Lei, W. Xiong, H. Gong, B. Xiao and N. Yan (2018). "Structure of the human voltage-gated sodium channel Nav1.4 in complex with beta1." Science 362(6412): eaau2486.
7. Lapointe, S., A. Perry and N. A. Butowski (2018). "Primary brain tumours in adults." Lancet 392(10145): 432-446.
8. Brown, R. H. and A. Al-Chalabi (2017). "Amyotrophic Lateral Sclerosis." N Engl J Med 377(2): 162-172.
Download full articles
 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!