 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| GFP-S453 | Rabbit | Polyclonal GFAP Antibody, Rabbit IgG | |||
| GFP-H5143 | Human | Human GFAP Protein, His Tag |  |
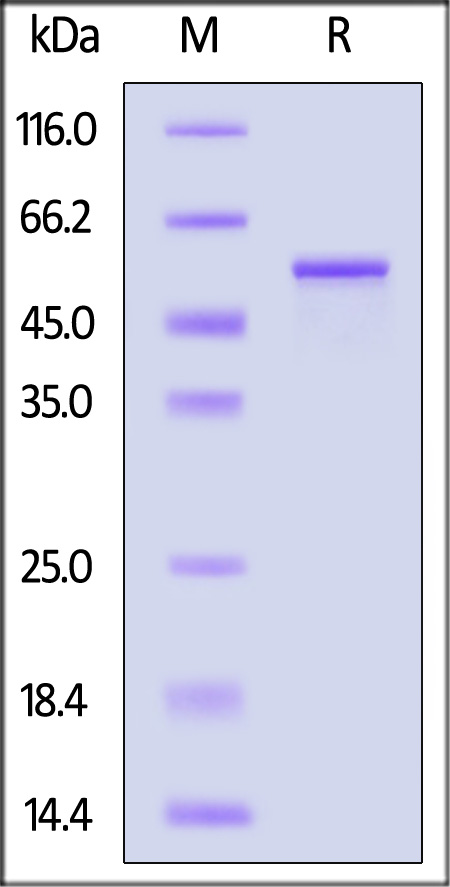
|
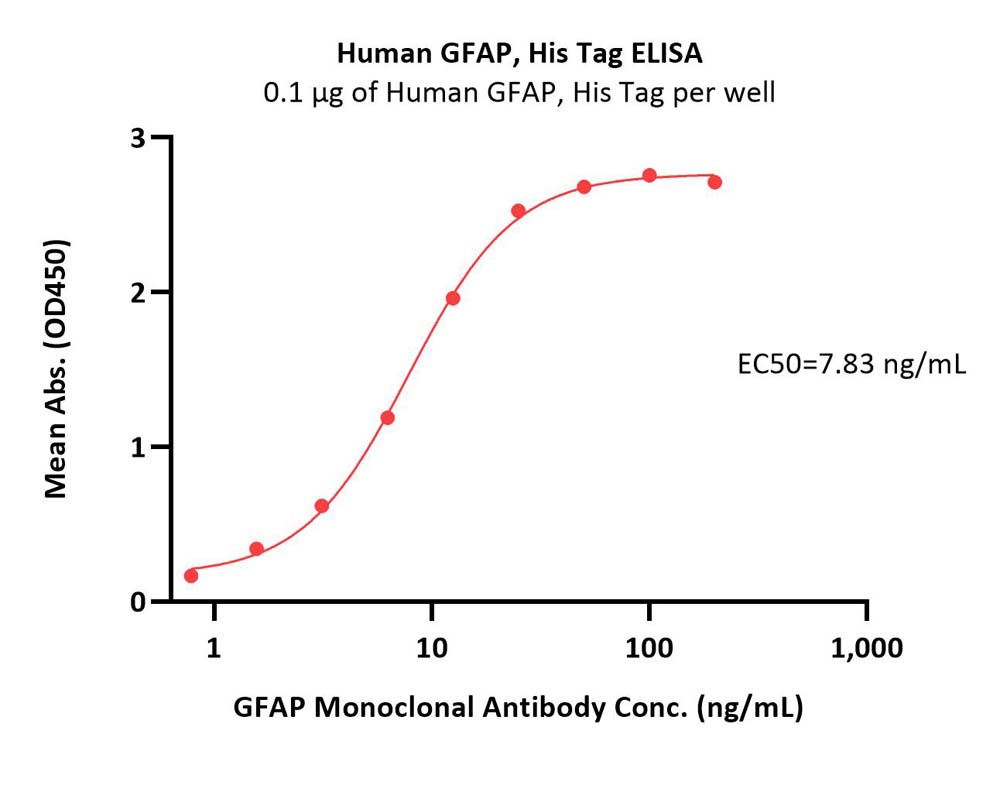
|
| GFP-M5148 | Mouse | Mouse GFAP Protein, His Tag |  |

|

|

Immobilized Mouse GFAP, His Tag (Cat. No. GFP-M5148) at 1 μg/mL (100 μL/well) can bind GFAP Monoclonal Antibody with a linear range of 0.4-25 ng/mL (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Gastrodine | Approved | Kpc Pharmaceuticals Inc | 辛基宁, 天眩清, 曲络彤, 丹彤 | Mainland China | Vertigo; Brain Injuries, Traumatic; Headache; Neurasthenia | Kpc Pharmaceuticals Inc | 2001-01-01 | Headache; Vertigo; Brain Injuries, Traumatic; Neurasthenia | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ION-373 | ION-373 | Phase 3 Clinical | Ionis Pharmaceuticals Inc | Alexander Disease | Details |
This web search service is supported by Google Inc.
