Click the following buttons and have a try.

ACROBiosystems Group,founded in 2010 and listed in 2021, is a biotechnology company aimed at being a cornerstone of the global biopharmaceutical and health industries by providing products and business models innovation. Our mission is to accelerate the progress of biopharmaceutical research and development to commercialization. To achieve this mission, our core values are established from a customer perspective by first satisfying our customers' needs for product quality. We have established a stringent quality management system to ensure our product quality and help our customers’ save time during drug development by meeting and exceeding customer expectations.
ACROBiosystems Group has established strict quality management systems according to ISO 9001:2015 and ISO 13485:2016 certifications. This is one of our strategic decisions to ensure that the quality of products and services is guaranteed through a third-party audited, efficient, and standardized system. Through this approach, we were able to systematically standardize the entire production process of our products including recombinant proteins, antibodies, enzymes, cytokines, kits, and other products. A clear organizational structure and department functions are also established, specifying the relevant responsibilities and authorities of each position and necessary interactions. This is all implemented to ensure that our quality policy and objectives are implemented and achieved as expected.
By adopting the PDCA cycle and risk-based thinking, we are able to proactively respond to market competition,and are continuously striving to improve our overall efficiency on the basis of understanding the needs and expectations of interested parties, so as to improve customer satisfaction.
Based on our quality policy to ‘First focus on customer needs and applicable statutory and regulatory requirements to be an important and valued partner in the field of targeted therapies’, ACROBiosystems group is willing to cooperate with customers and suppliers to emphasize a collaborative environment for therapy development. Regarding quality controls, we have strictly implemented a comprehensive quality control process starting from drug substance to finished products, which includes 20+ advanced testing techniques, 30+ tests, 4 audits and 3 releases .
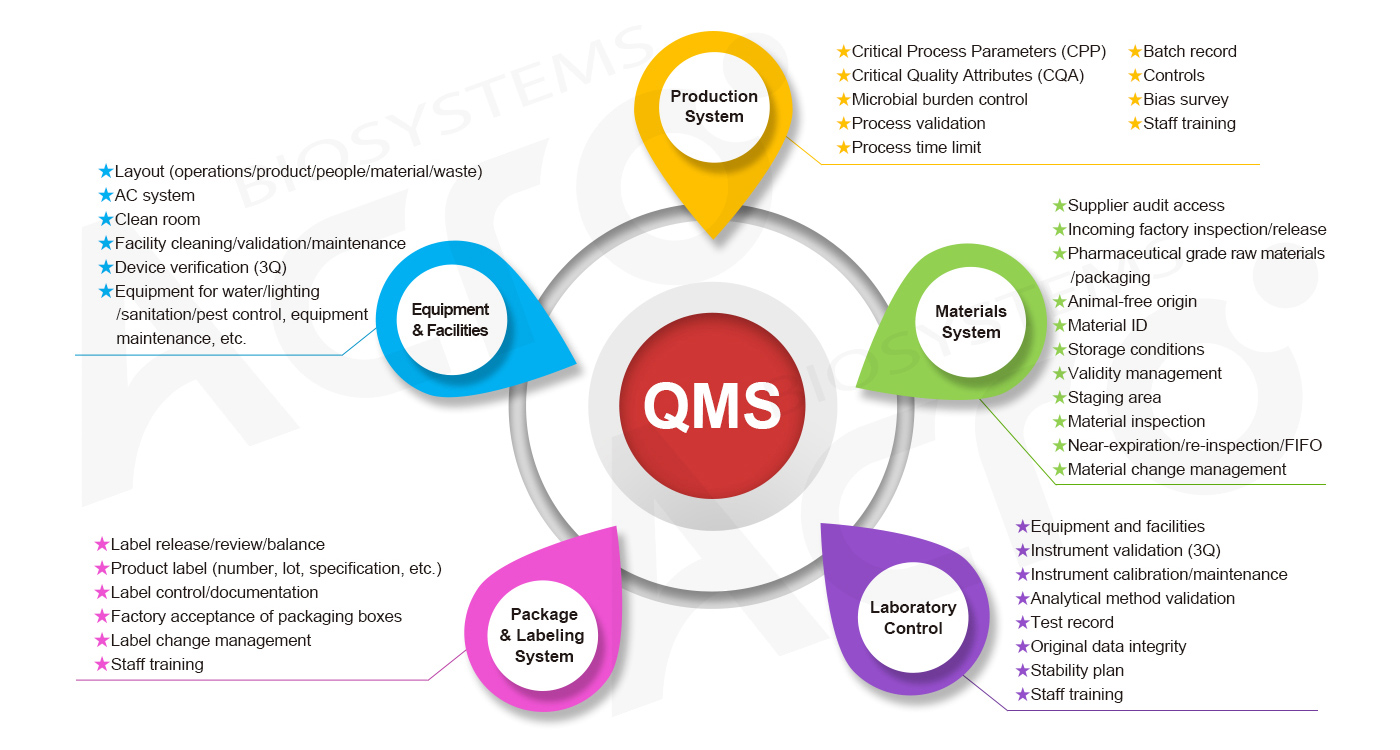
We launched GMP-grade products in early 2022, including cytokines, antibodies, enzymes, and magnetic beads for ex vivo cell culturing. These products are not only produced under a pharmaceutical-grade manufacturing plant, but are also designed and controlled under relevant domestic and international CGT regulations. Our products are produced under five interconnecting systems: material system, manufacturing system, facility/equipment system, packaging/labeling system, and laboratory quality control system. which comprehensively elaborates on the "true GMP ".
Click to learn about our GMP management system .
Law-abiding awareness
• the basis and premise of complying with the relevant national or regional laws and regulations
Customer focus
• Social awareness: the purpose and social value of our company come from whether customers recognize and are satisfied with our work
• Service awareness: to guide daily work, provide services and create value for customers from the needs and concerns of external and internal customers
• Market awareness: to design products and service procedures and carry out other activities from the perspective of customers' reception and usage habits when carrying out work or making decisions
Important partners in the industry
• Good profits and cash flows
• High operational efficiency and output
• Good brand awareness and customer relations
• Powerful support from our internal team
• Leading market dominance
Why Choose ACROBiosystems
Better Design
Application-oriented development strategies |  Over 95% of proteins are produced from HEK293 to ensure the native conformation of our proteins Over 95% of proteins are produced from HEK293 to ensure the native conformation of our proteins
 Six guaranteed technology platforms for multi-pass transmembrane proteins, next-generation fluorescent site-directed labeling, and enzymology Six guaranteed technology platforms for multi-pass transmembrane proteins, next-generation fluorescent site-directed labeling, and enzymology
 Dedicated research & development brands including ActiveMax, GENPower, ViruStop, etc. Dedicated research & development brands including ActiveMax, GENPower, ViruStop, etc.
 Custom products according to customer application requirements Custom products according to customer application requirements
|
Better Quality
Strict quality control systems |  Strict quality and production process control Strict quality and production process control
 Validated analytical methodologies Validated analytical methodologies
 DMF (FDA) filings for recombinant protein products DMF (FDA) filings for recombinant protein products
 ISO9001 and ISO13485 certified ISO9001 and ISO13485 certified
 GMP quality management system GMP quality management system
 CNAS-accredited SPR testing services available CNAS-accredited SPR testing services available
|
Better Support
24h Technical support and free resources |  24-hour technical support on weekdays 24-hour technical support on weekdays
 Free protocols on bioactivity validation Free protocols on bioactivity validation
 Open-access marketing information & training resources Open-access marketing information & training resources
 Resources for monitoring clinical progress and market dynamics Resources for monitoring clinical progress and market dynamics
 Comprehensive regulatory support documentation Comprehensive regulatory support documentation
 Extensive collaborations with our partners Extensive collaborations with our partners
|
Better Customer Service
Customers come first |  1 to 5-day global shipping 1 to 5-day global shipping
 Real-time, online support or local customer support available Real-time, online support or local customer support available
 Custom services available according to customer demands Custom services available according to customer demands
 Inventory reservation system to reserve the same batch or lot Inventory reservation system to reserve the same batch or lot
|


Since its establishment, ACROBiosystems Group has been improving its quality system for over 12 years, aiming to help customers accelerate the progress of drug development and its clinical applications. We passed ISO 9001:2015 Quality Management System Certification for the first time in 2016; passed ISO 13485:2016 Medical Device Quality Management Systems (QMS) Certification in 2019; established a GMP-grade product quality management system in 2021; and passed the CNAS accreditation regarding our SPR service in 2022. A good quality management system is continuously improved based on the evolving customer requirements, regulatory requirements, and market circumstances. ACROBiosystems has a professional quality management team, which has extensive experience and is intimately familiar with regulations related to biomedical sciences in countries and regions around the world. By closely following industry trends, our team can rapidly establish or modify the quality management system according to various product types, customer application scenarios, and regulatory requirements. This makes it feasible to develop and manufacture products that meet the requirements of different customers and ensure that the regulatory requirements and market circumstances are met.
ISO Quality Management System
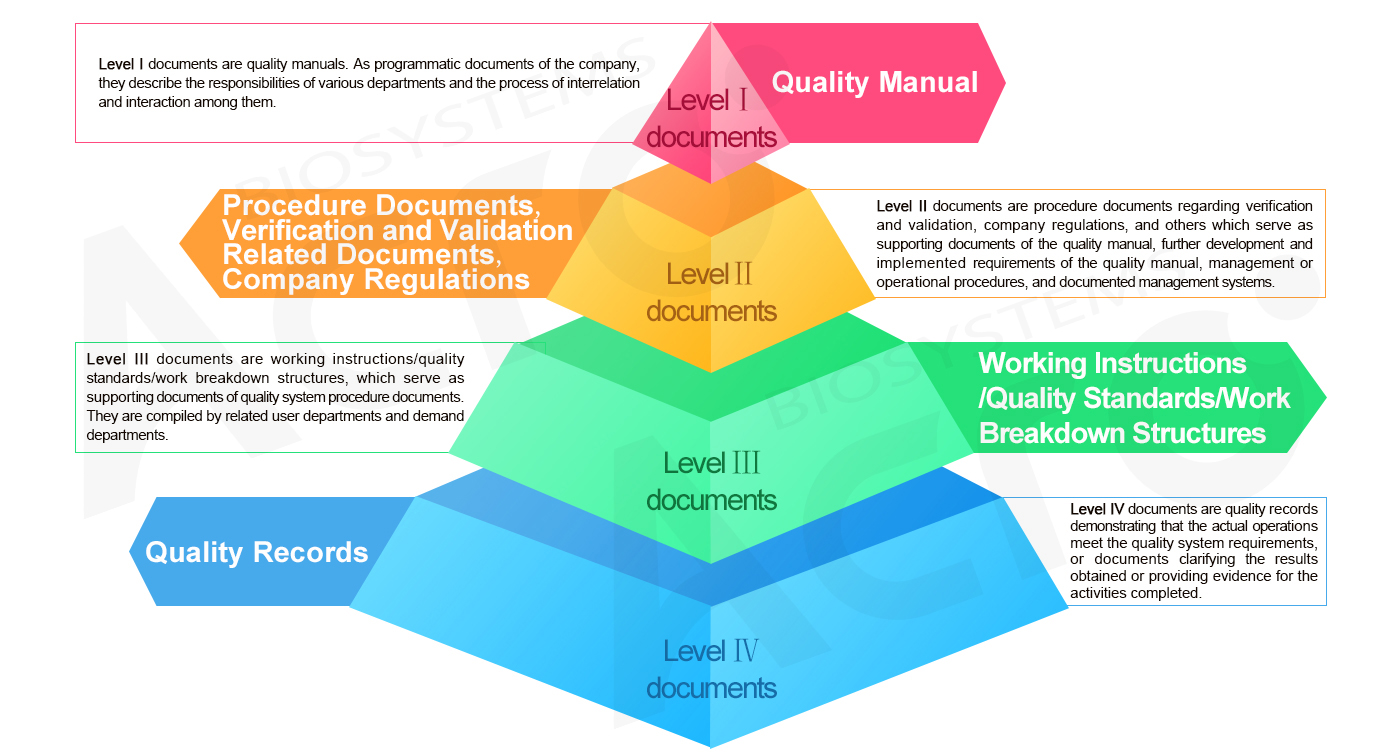
Document Management System of ACROBiosystems: Four-level "pyramid" Structure
Six Features of ACROBiosystems’ Quality Management System
ACROBiosystems has established a quality management system under the requirements of ISO 9001:2015 and ISO 13485:2016, and passed the QMS certification in 2016 and 2019, respectively. By running the system efficiently, we have ensured the management of the whole life cycle of products and services from design and development, bench-scale testing, formal production testing, marketing to customer feedback.
The risk-based thinking and design concept enables the quality system of ACROBiosystems to respectively establish management requirements for such system modules as Man (E-Learning online training system), Machine (verification and validation management system), Material (hierarchical management system of materials and suppliers), Method (four-level document structure), Environment (QA monitoring of production environment and process), and Measurement (20+ testing techniques, 30+ testing steps, 4 audits, and 3 releases), and QA personnel are specially assigned for each module. During the routine operation of the quality management system, ACROBiosystems applies multiple monitoring and measring means(routine testing/supplier performance/customer satisfaction survey/internal audit/management review/external audit, etc.) to discover the problems in real time and make improvements to ensure the effectiveness, adequacy, and suitability of our quality management system.
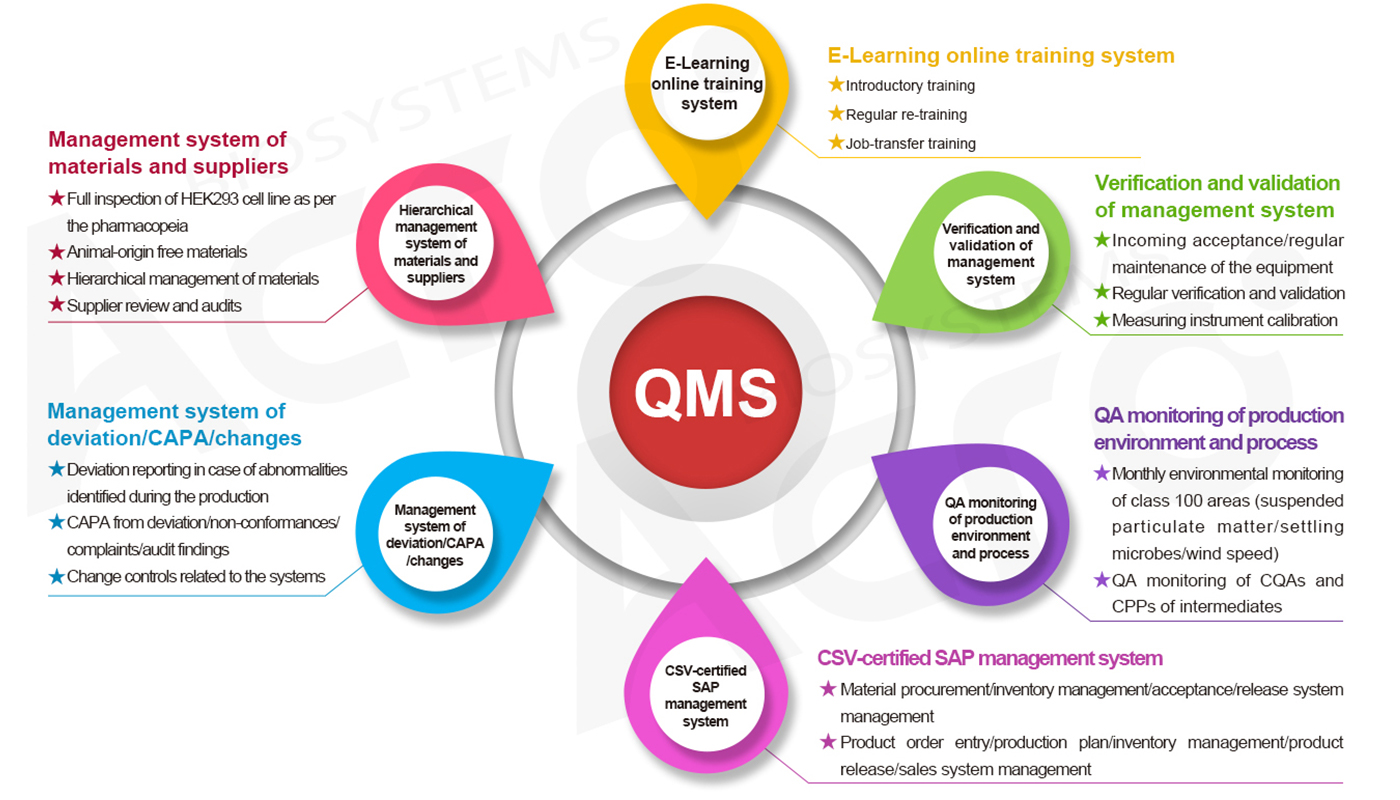
GMP Quality Management System — always under continuous improvement
Good Manufacturing Practice (GMP) is a set of standard rules formulated by the U.S. Food and Drug Administration (FDA) and adopted globally on personnel, premises and facilities, equipment, manufacturing management, quality management, and document management to produce high quality pharmaceuticals. As a set of compulsory standards applicable to pharmaceutical and other industries, GMP is the basic guideline for pharmaceutical manufacturing and quality management. It is applicable to all key processes that affect the finished product quality in the whole process of pharmaceutical manufacturing and in the production of drug substances. Pharmaceutical manufacturers are required to have verified production equipment, optimized manufacturing processes, a comprehensive quality management system, and a strict testing system. This ensures that the product quality meets relevant standards and to identify and solve problems occurring in the production process in a timely manner.
Throughout the whole process of pharmaceutical manufacturing, GMP controls the factors affecting the product quality through scientific approaches and effective measures to ensure that the quality of products consistently meets the manufacturing quality standards.
GMP, a set of requirements established to prevent (especially unknown) contamination, confusion and errors, is used to compensate for the limitations of final product testing.
To better support CGT-related customers, ACROBiosystems has established the GMP quality management system for GMP-grade products.
GMP Quality Management System of ACROBiosystems
Quality Management System
 Manufactured and QC tested under GMP compliance
Manufactured and QC tested under GMP compliance
 Designed under ISO 9001:2015 and ISO 13485:2016
Designed under ISO 9001:2015 and ISO 13485:2016
 Animal-Free materials
Animal-Free materials
 Materials purchased from approved suppliers
Materials purchased from approved suppliers
 ISO 5 cleanrooms and automatic filling equipment
ISO 5 cleanrooms and automatic filling equipment
 Qualified and well-trained personnel
Qualified and well-trained personnel
 Quality-related documents reviewed and approved by QA
Quality-related documents reviewed and approved by QA
 Fully batch production and control records
Fully batch production and control records
 Equipment maintenance and calibration
Equipment maintenance and calibration
 Validation of analytical procedures
Validation of analytical procedures
 Stability studies conducted
Stability studies conducted
 Comprehensive regulatory support files
Comprehensive regulatory support files
 Click here to applicate the
Click here to applicate the
Regulatory Support File (RSF)
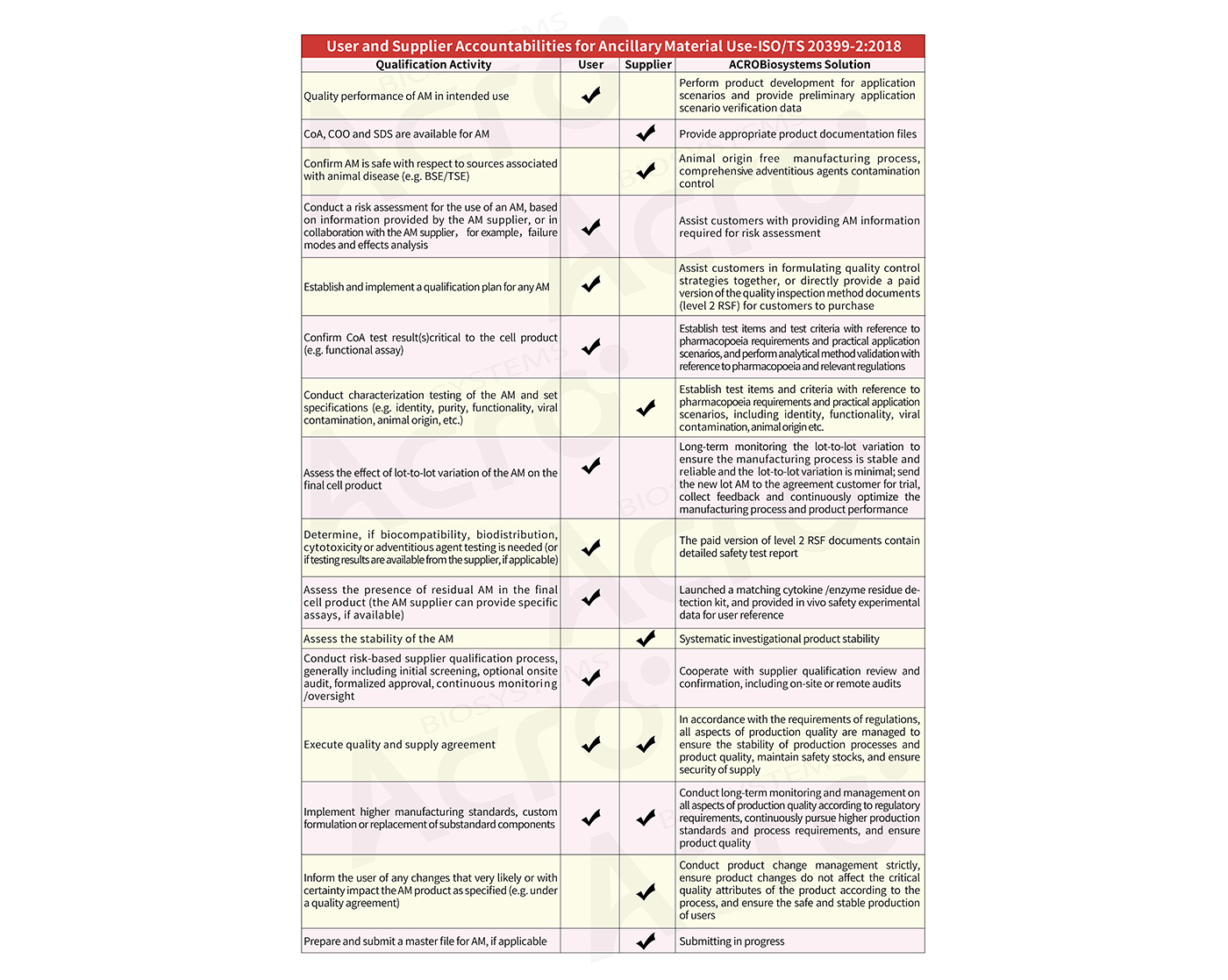
Production Qualification of Suppliers
 Suppliers should pass ISO quality system certification and have an effective quality management system.
Suppliers should pass ISO quality system certification and have an effective quality management system.
 Hardware facilities such as production workshops should strictly meet the requirements of GMP regulations and provide necessary documentation such as providing audit certificates from third-party authorities, such as drug production license, GMP audit reports from well-known third-party organizations, other qualification documents, etc.
Hardware facilities such as production workshops should strictly meet the requirements of GMP regulations and provide necessary documentation such as providing audit certificates from third-party authorities, such as drug production license, GMP audit reports from well-known third-party organizations, other qualification documents, etc.
 The suppliers should have advanced R&D and production technology, qualified manufacturing process and capabilities, and the capacity for continuous production and supply.
The suppliers should have advanced R&D and production technology, qualified manufacturing process and capabilities, and the capacity for continuous production and supply.

ISO 9001:2015 & ISO 13485:2016
Comprehensive Regulatory Support Files



"Good, structurally-defined protein products can reduce the uncertainty and lower the risk in every step of drug research and development.”
Research-use only Grade Products
ACROBiosystems, with its extensive experience in the development of target proteins, has summarized several key points of target proteins for drug development. The key points above mainly cover elements in five aspects:
★ Purity and Homogeneity
Purity testing methods commonly used by ACROBiosystems include SDS-PAGE, HPLC, and MALS.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE), is an analytical detection technique of proteins. It is mostly used to determine the proportion and content of target and miscellaneous proteins. However, since the proteins are denatured before analysis, it is hard to monitor the aggregation of proteins in solution.
MALS validation of purity is a key advantage of ACROBiosystems,which ensures correct protein structure, controllable protein aggregation state, homogeneity and batch-to-batch consistency. This is also the basis for consistent results of ELISA, SPR, and BLI activity validation. Therefore, we know that customers invested in biopharmaceutical development understand that the aggregation state and batch variation of proteins used are critical.
ACROBiosystems is committed to helping customers minimize the risks of drug research and development, ensuring purity levels for customers, and providing customers with high-quality recombinant proteins that are homogenous, consistent between batches, and more importantly, have the correct structure.
Click to read: Sharp-sighted SEC-MALS: Accurately determine the molecular weight and confirm the aggregation state of the protein
★ Confirming that the target protein is as close as possible to its natural conformation
As protein structure is the basis for functional activity, only proteins that are close to its natural conformation can properly perform and mimic its biological functions. In particular, the natural aggregation of proteins and correct formation of disulfide bonds are critical in maintaining the correct structure of proteins, and should be the main focus when developing of target proteins. ACROBiosystems selects HEK293 as the expression system of host cells, since proteins expressed by HEK293 system are better modified, including glycosylation modification, therefore closely resembling the natural conformation within human body. This cannot be achieved by other prokaryotic expression system or non-human eukaryotic system. As a critical raw material of antibody drug development, the high-quality target protein with natural conformation and correct structure can significantly increase the success rate of antibody drug development and reduce the uncertainty of drug development in critical application scenarios, such as antibodies acquisition by immunization, screening of functional antibodies and clinical validation of antibody effectiveness.
★ Activity and performance are validated in its target application scenarios
In the process of developing a target protein, it can be frequently observed that the same protein product performs completely differently under a real application scenarios. Therefore, it is necessary to establish the goals before the start of R&D and clarify the target application scenarios of the protein.
ACROBiosystems tests our protein under the target application scenario during the development process evaluates the protein performance under a simulated application scenario.
Read more: SPR&BLI: analysis platforms for protein binding activity 
★ Batch-to-batch consistency of products
ACROBiosystems products have high batch-to-batch consistency, which requires strict control of the protein manufacturing process under a well-established quality system. This ensures a high degree of consistency between product batches.
Click to view: Quality control process of ACROBiosystems products
★ Abilities to manufacture and continuously develop products
We maintain our protein products so they can be consistently produced over time, continuing consistent quality and supporting long-term demand for proteins within the development process and throughout the life cycle of biopharmaceuticals. ACROBiosystems has over 200 professionals working in product R&D to ensure that the capability of new product development is fully guaranteed.
GMP-Grade Products
ACROBiosystems interprets the relevant domestic and international regulatory requirements in depth, and formulates solutions for critical reagent-related problems that may be encountered in the development of cell therapy drugs.
>ACROBiosystems has summarized key features in the selection of GMP-grade raw materials for cell therapy based on domestic and international regulations and customer requirements to guide the development, manufacturing and quality control of products.
★ Production qualification of suppliers
● Suppliers should pass ISO quality system certification and have an effective quality management system.
● Hardware facilities such as production workshops should strictly meet the requirements of GMP regulations and provide necessary documentation such as providing audit certificates from third-party authorities, such as drug production license, GMP audit reports from well-known third-party organizations, other qualification documents, etc.
● The suppliers should have advanced R&D and production technology, qualified manufacturing process and capabilities, and the capacity for continuous production and supply.
Click to view: ACROBiosystems related qualifications
★ Product quality and performance
● Comprehensive analysis and characterization is performed, including identification, content, purity, biological activity, moisture, process-related impurities, potential contaminants, etc.
● The products are fully validated in practical application scenarios, and their performance can meet the application requirements (such as T cell culture and activation experiments).
● Each analytical procedure is systemically validated and traced, which is accurate, reliable and repeatable. Procedures can also be used for accurate analysis and verification of the product quality, along with long-term monitoring of the batch differences of manufacturing.
● The integrity of analytical data meets all regulatory requirements.
Click to view: GMP-grade products from ACROBiosystems
★ Batch-to-batch consistency and stability
● Production raw materials, plant equipment, laboratory analysis and testing, product packaging and storage meet the regulatory requirements of GMP quality management system and cell therapy raw materials.
● The key production parameters are strictly controlled, and the production process is stable and reliable.
● The batch differences of production are continuously monitored and analyzed, and OOS deviation analysis is performed.
● Product stability is fully evaluated to ensure stable and reliable performance during transportation, storage and use.
Click for quality control details
★ Product safety
● Our products undergo testing including sterility, bacterial endotoxin, virus, mycoplasma, related impurity content (HCP, DNA, antibiotics), final product residues, other miscellanies (foreign matter, additives, etc.)
Click to view: GMP quality inspection items of ACROBiosystems
★ Comprehensive Regulatory support documentation
● We provide a comprehensive set of documentation on safety, including proof of origin, Certificate of Analysis (COA), outsourced test report and related qualification certification, packing instructions, TSE/BSE-free statement, animal origin-free (AOF) statement, DMF documents, RSF regulatory support documents, etc.
Click to request an RSF document

Ensure that your products meet your needs with the most stringent quality control processes available

The Flow Chart of ACROBiosystems Product Quality Control
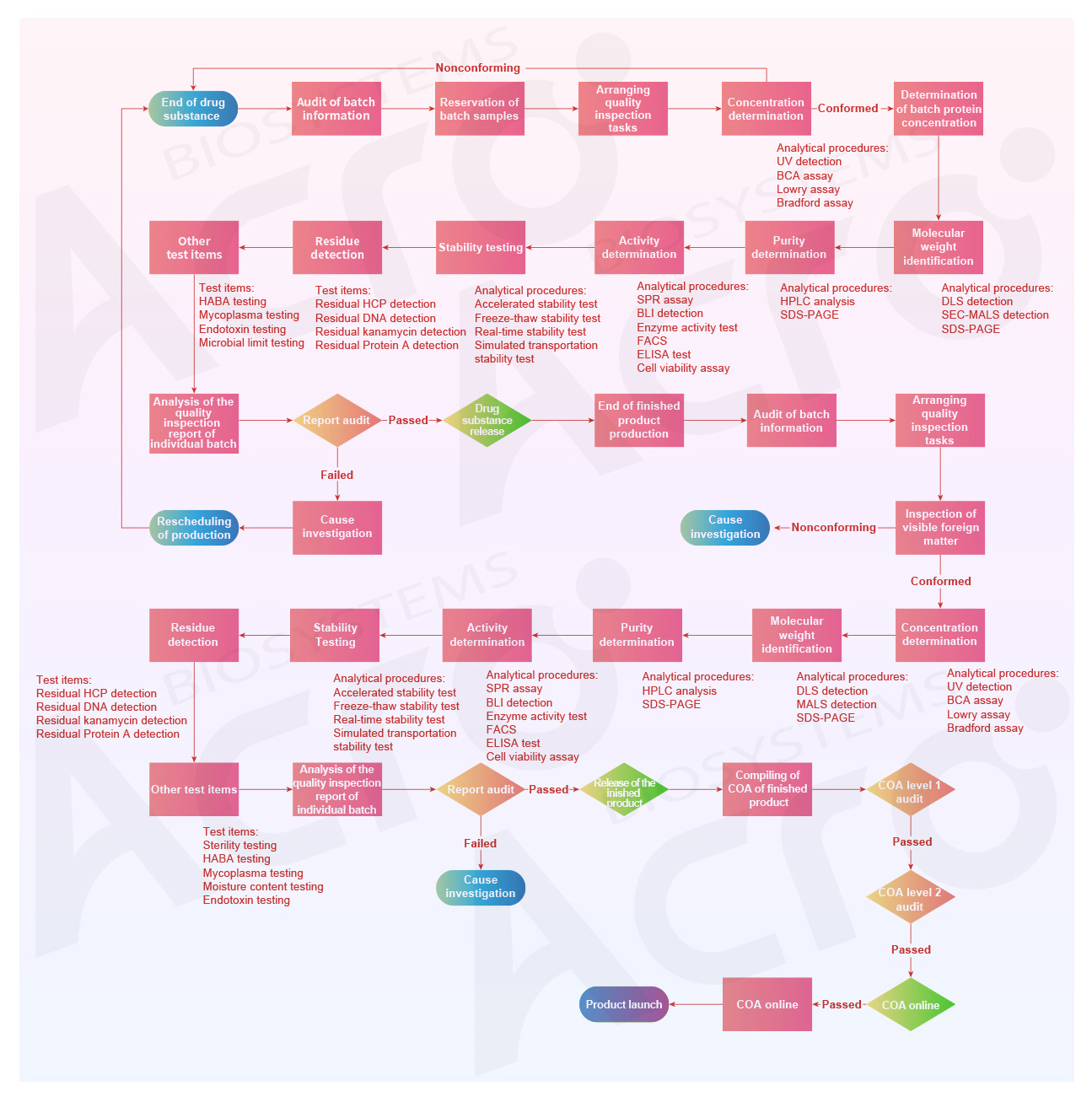
Comprehensive Quality Inspection
Well-established Stability Validation
Biological product stability is a critical part throughout the entire drug research and development, clinical trials, marketing and post-marketing quality study. This stability is the basis for the manufacturing, process, drug product formulation, packaging materials, storage, transportation conditions of the biological product. Real-time stability studies are the most reliable method to investigate product stability and determine product shelf life. However, this method is time-consuming, which greatly limits its use. Therefore, it is particularly important to find a more efficient method. The accelerated stability testing method based on Arrhenius equation is one of the recognized strategies. In recent years, more and more studies have proved its applicability and predictive accuracy.
ACROBiosystems products are validated for stability in four aspects: accelerated, freeze-thaw, real-time, and simulated transportation.
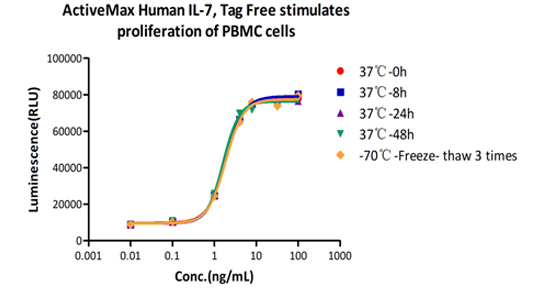
Accelerated stability:
The Cell based assay shows that ActiveMax Human IL-7 (Cat. No. IL7-H4219) is stable at 37°C for 48 hours.
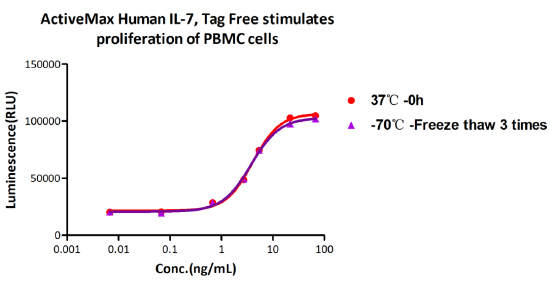
Freeze-thaw stability:
The Cell based assay shows that ActiveMax Human IL-7 (Cat. No. IL7-H4219) is stable at freezing and thawing 3 times.
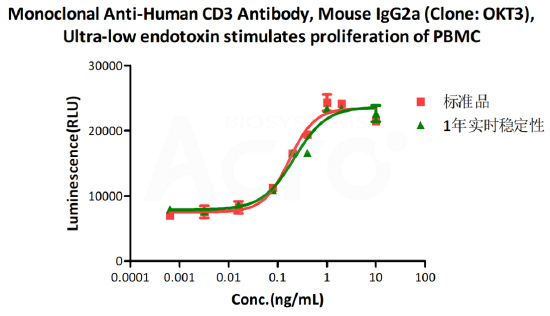
Real-time storage stability:
The Cell based assay shows that Monoclonal Anti-Human CD3 Antibody (Cat. No. CDE-M120a) is stable at 4°C for 1 year.
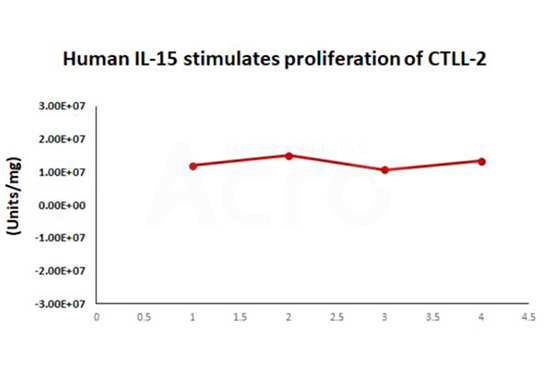
Simulated transportation stability:
The Cell based assay shows that Human IL-15 (Cat. No. GMP-L15H13) is stable at 37°C for 14 days.
Accurate Quantification
The biopharmaceutical development process often requires accurate concentrations of protein reagents used in experiments to avoid errors. As such, the loading quantity between batches should be kept strictly consistent, which puts forward higher requirements for the accurate quantification of protein reagents. Meanwhile, strict method validation is also important.
According to ICH Q2 and Chinese Pharmacopoeia standards for different proteins, we use the relevant standards for traceability as well as a comprehensive method validation. The results of repeatability show that the CV% of the same method performed by different operators on different days was less than 3%, and the recovery rates ranged from 90% to 108%, ensuring quantitative accuracy.
Traceability data for analytical procedures:
| Validation item | Test method | Traceability method |
|---|
| Validation criteria | Validation result | Validation criteria | Validation result |
|---|
| Repeatability | RSD≤3% | 0% | RSD≤3% | 1% |
| Different analysts on the same day | RSD≤3% | 0% | RSD≤3% | 3% |
| Same analyst on different days | RSD≤3% | 0% | RSD≤3% | 3% |
| Accuracy | 90%-108% | 100%-106% | 90%-108% | 97%-103% |
| Robustness | RSD≤3% | 0% | RSD≤10% | <10% |
| Linearity and range | R2>0.999 | 0.9996 | R2>0.999 | 0.9999 |
| Specificity | / | / | 90-110% | 96% |
There are many methods for protein quantification, such as UV spectrophotometry (UV 280), Folin-phenol method (Lowry method), BCA method, Bradford method, etc. These methods are based on different principles, and have their own advantages and limitations. For example, as long as the molar extinction coefficient is calculated according to the amino acid sequence, the UV absorption method can be used for fast protein quantification. It is a simple method which does not require special instruments, equipment, or training, making it the most used method in most laboratories. However, UV absorption method is susceptible to the interference of UV-absorbing impurities, such as residual trace nucleic acids, resulting in false high protein concentrations and batch differences caused by inconsistent quantification.
ACROBiosystems addresses these issues by adopting various quantification methods including UV absorption and BCA (modified Folin-phenol method) during the quantification of each batch of protein drug substance and finished product. Separate quantitative standards and standard batches for each protein product are also established. This fundamentally avoids the error of protein quantification caused by the limitations of any single method, and ensures the consistency of quantification among different batches of the same product, providing customers with the minimal batch-to-batch differences.
Purity/Molecular Weight Quality Control
To meet the needs of biopharmaceutical customers for high-purity protein, ACROBiosystems uses various methods including SDS-PAGE, SEC-HPLC, SEC-MALS, DLS to test the purity and homogeneity of products.
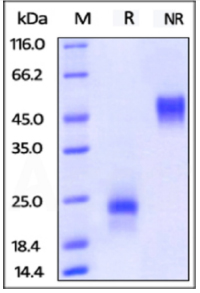
SDS-PAGE:
ActiveMax® Human VEGF165, Tag Free (MALS verified) on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 98%.
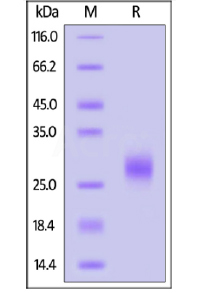
SDS-PAGE:
Human IL-7 on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.
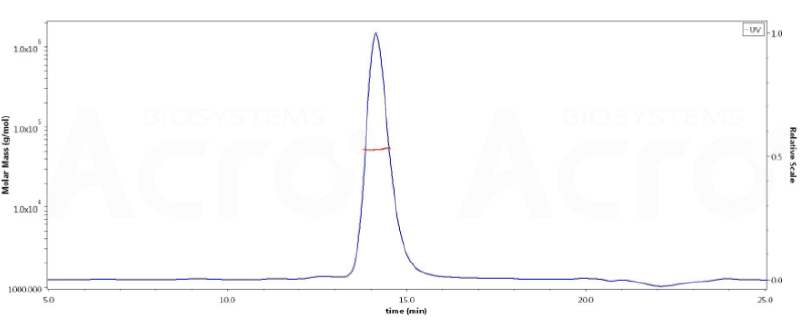
The SEC-HPLC & SEC-MALS data:
The purity of ActiveMax® Human VEGF165, Tag Free (MALS verified) (Cat. No. VE5-H4210) is more than 95% in HP-SEC, and the molecular weight of this protein is around 40-55 kDa verified by SEC-MALS.
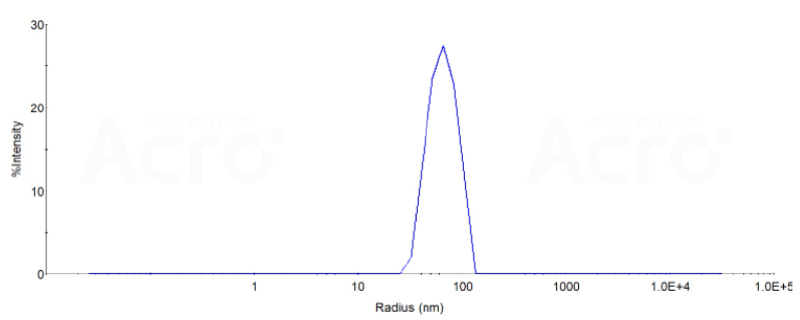
The DLS data:
The mean peak Radius of VLP is 60-80 nm with more than 95% intensity as determined by dynamic light scattering (DLS).
Click to read: Sharp-sighted SEC-MALS: Accurately determine the molecular weight and confirm the aggregation state of the protein
Multiplatform Bioactivity Testing
The bioactivity testing of drugs is performed throughout the R&D and production of biopharmaceuticals. In order to better help customers in the biopharmaceutical field, ACROBiosystems has established multiple bioactivity testing platforms, including ELISA, SPR, flow cytometry and cell-based assays, to provide customers in the biopharmaceutical field with better products of more consistent quality and referable bioactivity analytical protocols.
ELISA
Immobilized Human IL-17 RE (155-454), Fc Tag (Cat. No. ILE-H5256) at 2 μg/mL (100 μL/well) can bind Biotinylated Mouse IL-17C, His,Avitag (Cat. No. ILC-M82E9) with a linear range of 0.2-20 ng/mL (QC tested).
ELISA
Immobilized Human IL-15, Tag Free (Cat. No. IL5-H4117) at 5 μg/mL (100 μL/well) can bind Human IL-2 R beta, Fc Tag (Cat. No. ILB-H5253) with a linear range of 5-156 ng/mL (Routinely tested).
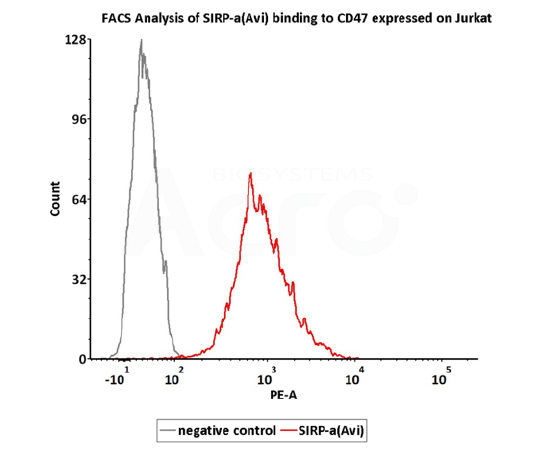
FACS
FACS assay shows that Biotinylated Human SIRP alpha, Fc,Avitag (Cat. No. CDA-H82F2) can bind to Jurkat cell expressing CD47. The concentration of SIRP alpha used is 3 μg/mL (QC tested).
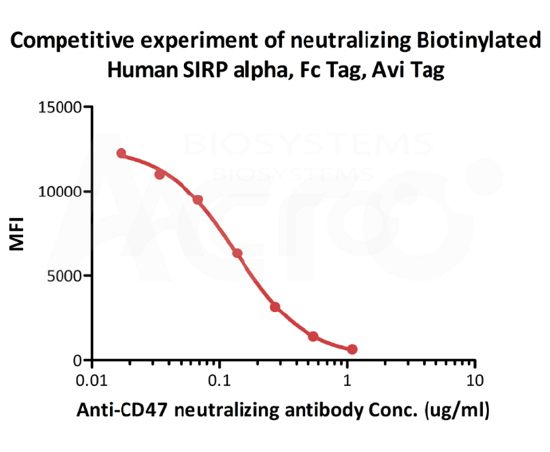
FACS
FACS analysis shows that the binding of Biotinylated Human SIRP alpha, Fc,Avitag (Cat. No. CDA-H82F2) to Jurkat expressing CD47 was inhibited by increasing concentration of neutralizing Anti- Human CD47 antibody. The concentration of SIRP alpha used is 1 μg/mL. IC50=0.1303 μg/mL (Routinely tested).
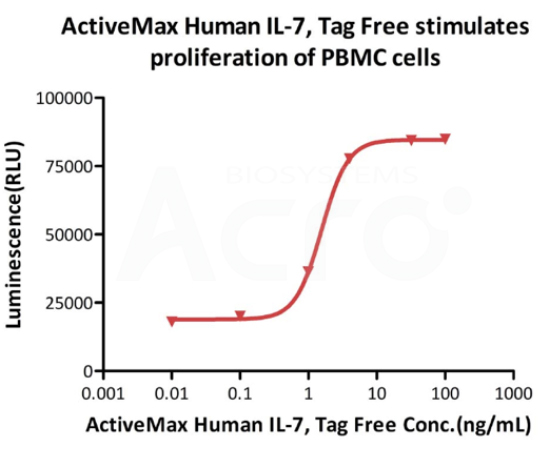
Cell based assay
ActiveMax Human IL-7, Tag Free (Cat. No. IL7-H4219) stimulates proliferation of PHA-P-activated human peripheral blood mononuclear cell (PBMC). The EC50 for this effect is 1.565 ng/mL, corresponding to a specific activity of > 1.0 ⅹ10^8 IU/mg, which is calibrated against human IL-7 WHO International Standard (NIBSC code: 90/530) (QC tested).
SPR
Human IL-2 R beta Protein, His Tag (Cat. No. CD2-H5221) captured on CM5 chip via anti-His antibody, can bind Human IL-15, premium grade (Cat. No. IL5-H4117) with an affinity constant of 21.7 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
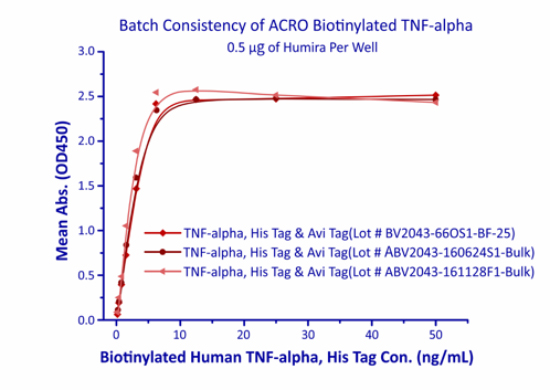
Batch Release
In order to ensure product quality and high batch-to-batch consistency, ACROBiosystems adopts the batch inspection and release strategy. Each new batch is compared to the corresponding reference standard, and only the batch of the equivalent quality level to the standard will be approved for release. Moreover, each batch of products is supplied with its own quality control report.
In the above ELISA analysis, three different lots of biotinylated hTNF-alpha (Cat. No. TNA-H82E3) were used detect immobilized Adalimumab (5ug/ml). The result showed that the batch variation among the tested samples is negligible.
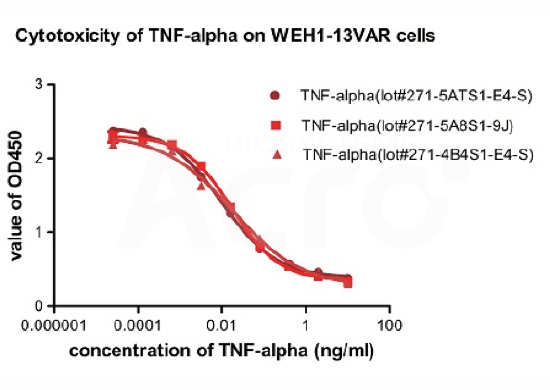
Recombinant Human TNF-alpha (Cat. No.TNA-H4211) induces cytotoxicity effect on the WEH1-13VAR cells in the presence of the metabolic inhibitor actinomycin D. The ED50 for this effect is 0.007-0.014ng/ml. The result shows that the batch variation among the tested samples is negligible.
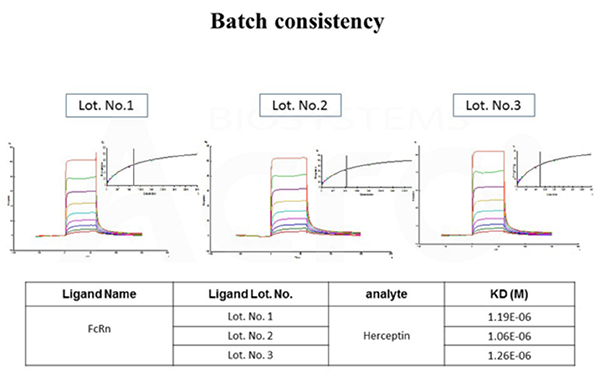
The binding affinity between Herceptin and different batches of FcRn / FCGRT & B2M Heterodimer Protein (Cat. No. FCM-H5286) were determined by SPR assay. The result shows that the batch variation among the tested different lots is negligible.
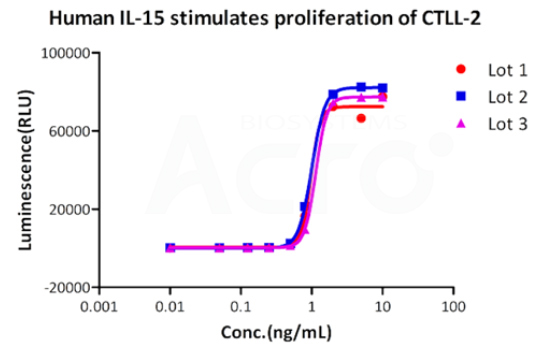
Bioactivity of three different lots of GMP Human IL-15 (GMP-L15H13) verified by cell-based assay, and the result shows very high batch-to-batch consistency.
Validation of Analytical Method
The use of accurate and credible analytical methods for testing is the basis for strict quality control of the product. For GMP grade proteins, we have completed sufficient analytical method validation according to ICH Q2 (R1) and Guidelines for Validation of Analytical Procedures in Chinese Pharmacopoeia to ensure the accuracy of the test results.
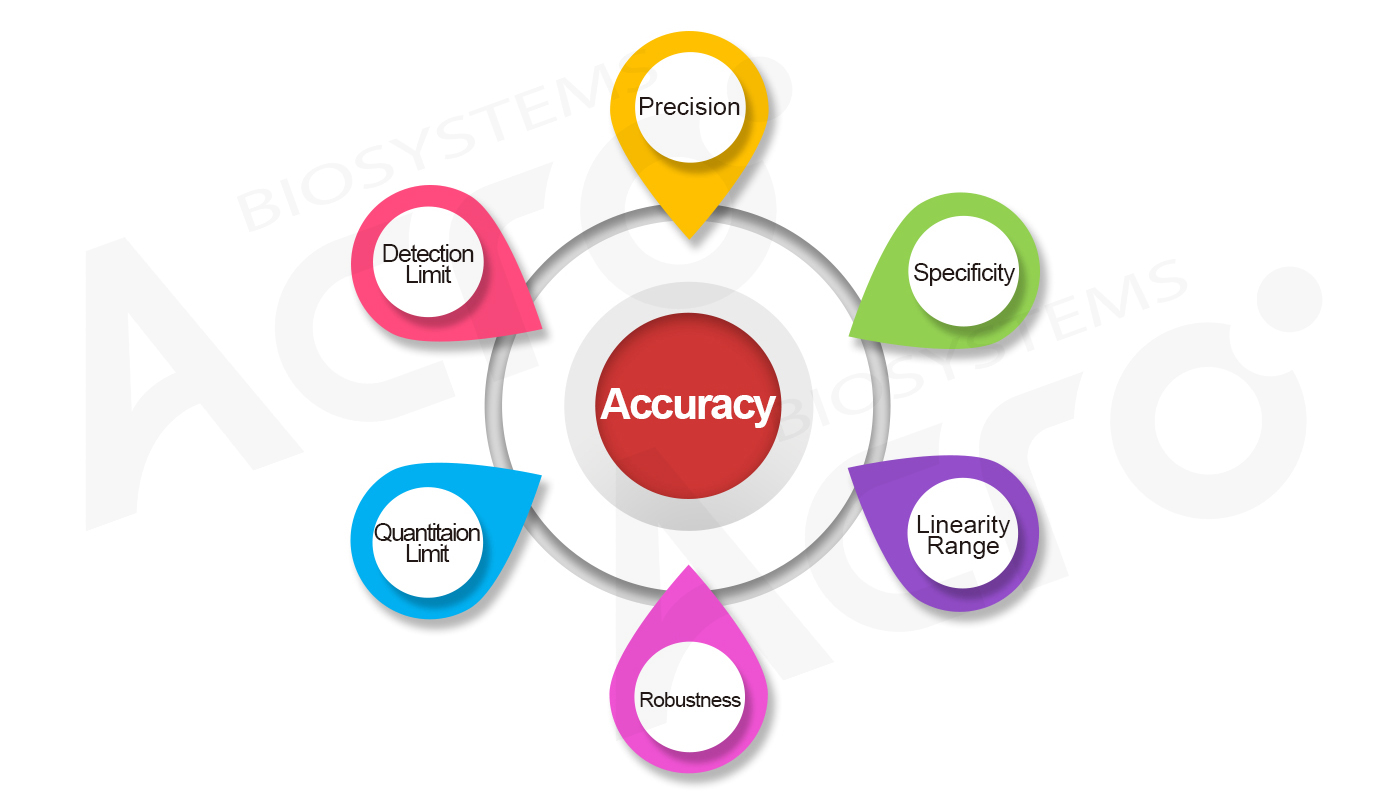
| Analytical Procedure | Test items | Source of procedures | Validation items |
|---|
| Lowry | Protein content | ICH Q2 (R1) and 9101 Guidelines for Validation of Analytical Procedures in Volume IV of ChP 2020 | specificity, accuracy, precision, linearity, range and robustness |
| SDA-PAGE | Identity | ICH Q2 (R1) and 9101 Guidelines for Validation of Analytical Procedures in Volume IV of ChP 2020 | specificity, accuracy, limit of detection (LOD), robustness |
| Endotoxins | Biological assay methods | Kinetic Chromogenic Assay in General Chapter 1143 Bacterial Endotoxins Test in Volume IV of ChP 2020 | reliability test of standard curve, and interference test of test sample |
| Sterility | Biological assay methods | Sterility Test in General Chapter 1101 Microbiological Examination in Volume IV of ChP 2020 | sensitivity test of culture medium, and method suitability test |
| Mycoplasma | Biological assay methods | ICH Q2 (R1) and 9101 Guidelines for Validation of Analytical Procedures in Volume IV of ChP 2020 | specificity, accuracy, precision, limit of quantitation (LOQ), linearity, range and robustness |
| DNA residues | Impurity determination/ Quantification | ICH Q2 (R1) and 9101 Guidelines for Validation of Analytical Procedures in Volume IV of ChP 2020 | specificity, accuracy, precision, limit of quantitation (LOQ), linearity, range and robustness |
| Cell Viability | Biological assay methods | 9401 Guidelines for Biological Activity/Potency Assay Validation of Biological Products in Volume IV of ChP 2020 | specificity, relative accuracy, intermediate precision, linearity, range |
| HCP Residues | Impurity determination/ Quantification | ICH Q2 (R1) and 9101Guidelines for Validation of Analytical Procedures in Volume IV of ChP 2020 | specificity, accuracy, precision, limit of quantitation (LOQ), linearity, range and robustness |
| Kanamycin Residues | Impurity determination/ Quantification | ICH Q2 (R1) and 9101 Guidelines for Validation of Analytical Procedures in Volume IV of ChP 2020 | specificity, accuracy, precision, limit of quantitation (LOQ), linearity, range and robustness |
Table 1. Example of the validation of cell viability analytical procedure
| Validation item | Validation criteria | Validation result | Validation conclusion |
|---|
Specificity | Negative reaction, interference ≤ 20%. | average RSD = 5%
maximum RSD = 12% | Conformed |
Relative accuracy | The relative bias should be within ± 12%. | The maximum bias was 6%. | Conformed |
The slope of regression equation should range from 0.80 to 1.25. | The slope was 1.11. |
Intermediate precision | The geometric coefficient of variation (GCV, %) of relative potency of each potency level measured by different analysts on different dates should not be greater than 20%. | a±GCV15% | Conformed |
Linearity | The correlation coefficient of linear regression equation should not be less than 0.95. | 0.96 | Conformed |
Range | It should cover the range of potency quality standard of the product. | This method covered 64%–156% potency levels. | Conformed |
Table 2. Example of precision validation of the enzyme activity testing
| Test date | Number of detections | Measured value | Different analysts on the same day
RSD | Employee A, same analyst on different days
RSD | Employee B, same analyst on different days
RSD | Employee A, same-day repeatability
RSD | Employee B, same-day repeatability
RSD | Different analysts on different days
RSD |
|---|
| EmployeeA | EmployeeB |
|---|
| DAY 1 | 1 | 1.49 | 1.52 | 1% | 5% | 4% | 2% | 3% | 6% |
2 | 1.46 | 1.48 | 1% |
3 | 1.52 | 1.52 | 0% |
4 | 1.49 | 1.47 | 1% |
5 | 1.45 | 1.40 | 3% |
6 | 1.51 | 1.51 | 0% |
DAY 2 | 1 | 1.36 | 1.56 | 9% | 2% | 3% |
2 | 1.33 | 1.53 | 10% |
3 | 1.39 | 1.60 | 10% |
4 | 1.39 | 1.58 | 9% |
5 | 1.32 | 1.52 | 10% |
6 | 1.39 | 1.61 | 11% |

1. What are the quality controls before product release?
ACROBiosystems has built a strict quality control system. For quality control, we have strictly implemented a comprehensive quality control process from drug substance to finished products, which consists of 20+ advanced testing techniques, 30+ tests, 4 audits and 3 releases.

Click to view: Quality control process of ACROBiosystems
2. What is DMF? Is there an approval certificate for DMF archiving? Has ACROBiosystems applied for the DMF filing of its products with FDA? What are the products submitted for DMF filing?
(1) DMF is the abbreviation of "Drug Master File". It is a complete set of documents including product chemistry, production and controls (CMC) information. The contents include general product information, and the information and data on manufacturing process, impurity research, stability, etc. It also includes core secrets related to the product, such as detailed process description, critical process parameters, complete material information, etc. There are two states of DMF: "A" = Active state, which indicates that the DMF has been assigned with a filing number/registration number/record number and is normally maintained and available. "I" = Inactive state, indicating that the DMF has been closed by the holder or FDA and is unavailable.
(2) Currently, there is no certificate but it will be published on the FDA website and accessible to all.
(3) Yes, ACROBiosystems achieved the first DMF filing of recombinant protein reagent in July 2020, and is the first company in the industry of which the recombinant protein has passed the DMF filing of FDA.
(4) The products of 33 catalog numbers have been submitted for DMF filing, and more products will continue to be submitted for DMF filing in the future.Click for more DMF filing details.
If you require IND/BLA application, please contact quality@acrobiosystems.com,to obtain a letter of authorization (LOA). In addition, ACROBiosystems expects to complete the DMF filing of all GMP products by mid-2022.
3. How does ACROBiosystems ensure product quality?
Product quality control is a comprehensive control system. The control strategies of ACROBiosystems include: control and inspection of raw materials; testing of intermediate products, semi-finished products and final products according to standardized SOPs. Thus, the product quality is ensured to be effectively controlled and guaranteed from beginning to end. Product release is conducted by qualified authorized personnel, and supplier audits are conducted as needed. In addition, the effectiveness of quality control is also monitored by monitoring product complaints and nonconforming products, corrective and preventive actions and plans, etc.
4. What is the difference between GMP and Premium grades?
| Premium Grade | GMP Grade |
|---|
| Application | Research and Development; Preclinical research and transition into clinical phases. | Designed to meet clinical phase requirements and bolster your IND application to various regulatory bodies. v |
| Quality System | ISO 9001 and 13485 Quality Management System | ISO 9001 and 13485 Quality Management System (R&D stage) including GMP Quality Management System (Production stage) |
| Production | ISO certified Workshop | GMP grade workshop certified by third-party audits |
| Transient, stable cell lines | Stable cell lines (Comprehensive external inspections) |
| Mostly animal-origin free materials | Animal-origin free materials |
| Pharmaceutical-grade materials | Pharmaceutical-grade materials |
| Strict secondary sterilization filtration | Strict secondary sterilization filtration |
| Laminar flow cleanroom with manual fill finish | B+A grade cleanrooms with automated fill finish |
| No specific virus removal or inactivation process | Specific virus removal / inactivation process (nanofiltration + low pH) |
| Quality Control | Sterility / Mycoplasma testing | Sterility / Mycoplasma testing |
| Endotoxin control and detection | Endotoxin control and detection |
| Validated key production equipment and analytical instruments | Strict verification, auditing, and tracking of all equipment and methods. |
| Process-related impurity testing (DNA, HCP, Residue) | Process-related impurity testing (DNA, HCP, Residue) |
| No additional quality control tests | Comprehensive virus residue testing, animal in vivo safety experiments |
| Documentation | Minimum documentation and certifications | Comprehensive regulatory support documentation |
| DMF files (Few products) | DMF files (All products) |
 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!




![]() Manufactured and QC tested under GMP compliance
Manufactured and QC tested under GMP compliance![]() Designed under ISO 9001:2015 and ISO 13485:2016
Designed under ISO 9001:2015 and ISO 13485:2016![]() Animal-Free materials
Animal-Free materials![]() Materials purchased from approved suppliers
Materials purchased from approved suppliers![]() ISO 5 cleanrooms and automatic filling equipment
ISO 5 cleanrooms and automatic filling equipment![]() Qualified and well-trained personnel
Qualified and well-trained personnel![]() Quality-related documents reviewed and approved by QA
Quality-related documents reviewed and approved by QA![]() Fully batch production and control records
Fully batch production and control records![]() Equipment maintenance and calibration
Equipment maintenance and calibration![]() Validation of analytical procedures
Validation of analytical procedures![]() Stability studies conducted
Stability studies conducted![]() Comprehensive regulatory support files
Comprehensive regulatory support files
![]() Suppliers should pass ISO quality system certification and have an effective quality management system.
Suppliers should pass ISO quality system certification and have an effective quality management system.![]() Hardware facilities such as production workshops should strictly meet the requirements of GMP regulations and provide necessary documentation such as providing audit certificates from third-party authorities, such as drug production license, GMP audit reports from well-known third-party organizations, other qualification documents, etc.
Hardware facilities such as production workshops should strictly meet the requirements of GMP regulations and provide necessary documentation such as providing audit certificates from third-party authorities, such as drug production license, GMP audit reports from well-known third-party organizations, other qualification documents, etc.![]() The suppliers should have advanced R&D and production technology, qualified manufacturing process and capabilities, and the capacity for continuous production and supply.
The suppliers should have advanced R&D and production technology, qualified manufacturing process and capabilities, and the capacity for continuous production and supply.