Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Novel Drug Development Opportunities in Autoimmune Diseases 
Ocular SystemRespiratory SystemNervous SystemDigestive SystemCardio-vascular SystemUrinary SystemEndocrine SystemHematological SystemReproductive SystemSkin & Connective Tissue SystemMuscular SystemSkeletal & Joint System
Autoimmune diseases are a group of debilitating conditions characterized by the body's immune system mistakenly attacking its own healthy cells, tissues, and organs. This immunological dysregulation stems from a breakdown in self-tolerance mechanisms, resulting in an aberrant immune response that causes inflammation and tissue damage.
These diseases can be classified into two main types based on the range of organs and tissues affected:
Organ-Specific Autoimmune Diseases: These disorders target a single organ or tissue type, such as Multiple Sclerosis, Autoimmune Hepatitis, and Type 1 Diabetes Mellitus.
Systemic Autoimmune Diseases: In this category, the autoimmune assault extends to multiple organs and systems. Prominent diseases include Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis, Ankylosing Spondylitis, Sjogren's Syndrome, Polymyositis, and Dermatomyositis.
At ACROBiosystems, we are committed to providing comprehensive products and solutions to accelerate autoimmune disease drug discovery and development. Our innovative product portfolio encompasses high-quality recombinant proteins, functional cell lines, antibodies, and testing kits autoimmune disease targets. These products and solutions are designed to facilitate various stages of the biological drug development pipeline, empowering researchers to advance promising therapies efficiently.
Autoimmune Diseases Guide
Hot Products in Autoimmune Disease Drug Development
Reference
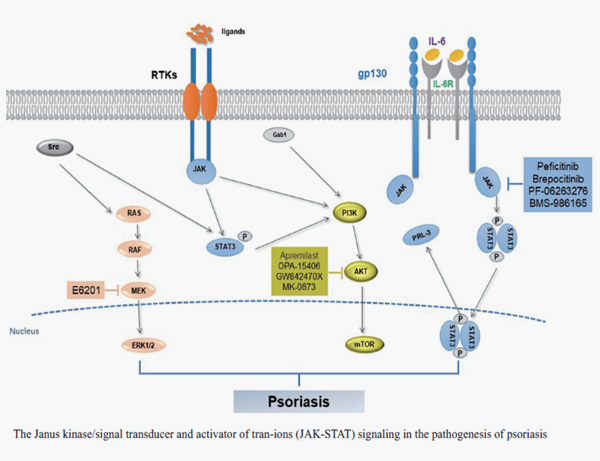
1. ZHU Yu-yu, SONG Cheng-lin, SUN Yang. Advances in mechanisms for psoriasis and drug regulation. Acta Pharmaceutica Sinica, 2020, 55(7): 1393-1400.
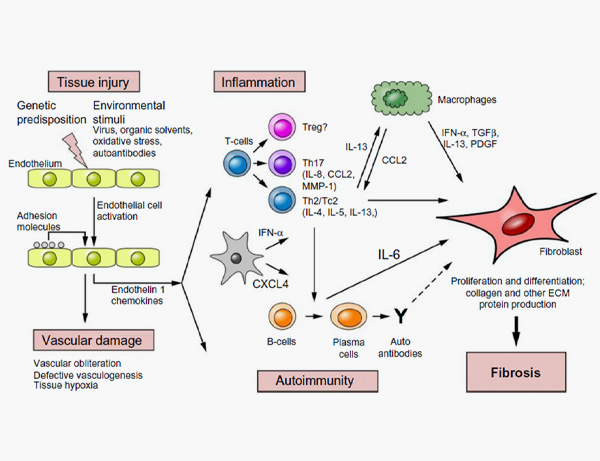
2. Fuschiotti P. Current perspectives on the immunopathogenesis of systemic sclerosis. Immunotargets Ther. 2016;5:21-35 .
3. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003 Sep 20;362(9388):971-82.
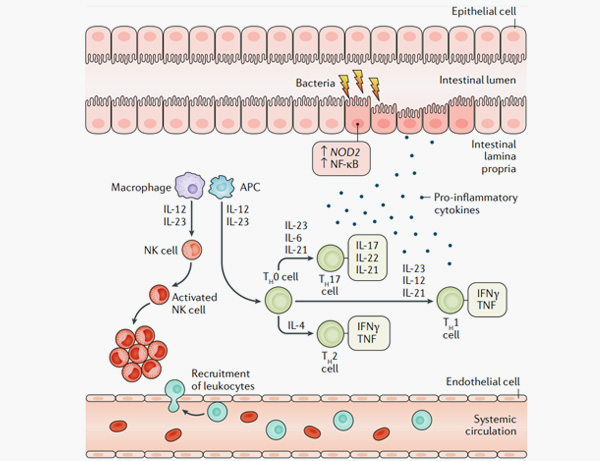
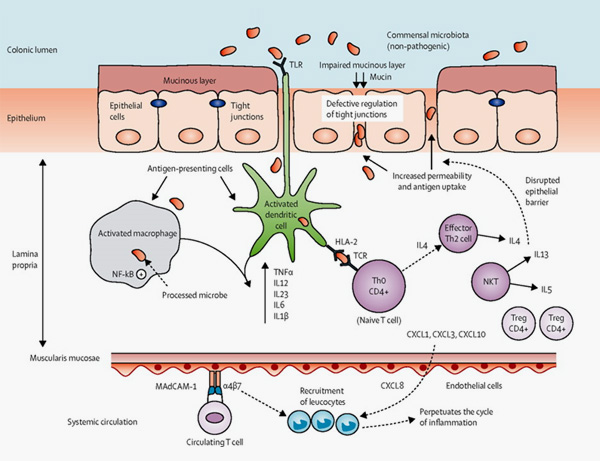
4. Roda, G., Chien Ng, S., Kotze, P.G. et al. Crohn’s disease. Nat Rev Dis Primers 6, 22 (2020).
5. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012 Nov 3;380(9853):1606-19.
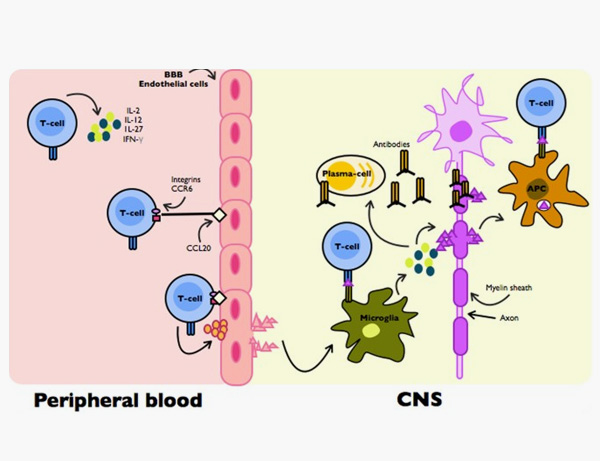
6. Cervantes-Gracia, K., Husi, H. Integrative analysis of Multiple Sclerosis using a systems biology approach. Sci Rep 8, 5633 (2018).
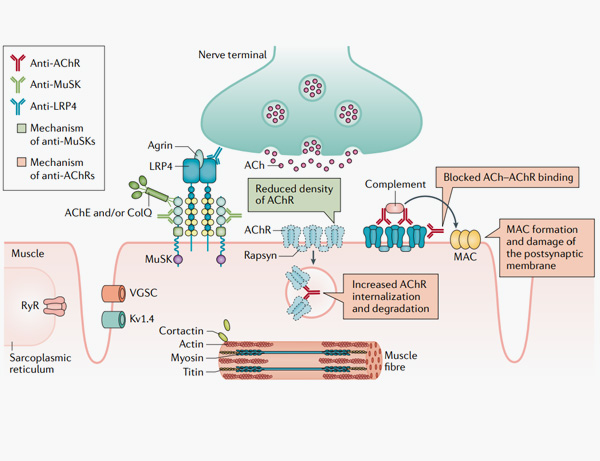
7. Gilhus, N.E., Tzartos, S., Evoli, A. et al. Myasthenia gravis. Nat Rev Dis Primers 5, 30 (2019).
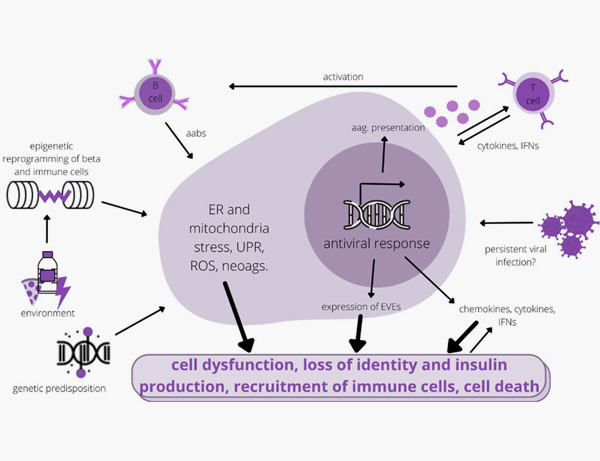
8. Zajec A, Trebušak Podkrajšek K, Tesovnik T, Šket R, Čugalj Kern B, Jenko Bizjan B, Šmigoc Schweiger D, Battelino T, Kovač J. Pathogenesis of Type 1 Diabetes: Established Facts and New Insights. Genes (Basel). 2022 Apr 16;13(4):706.
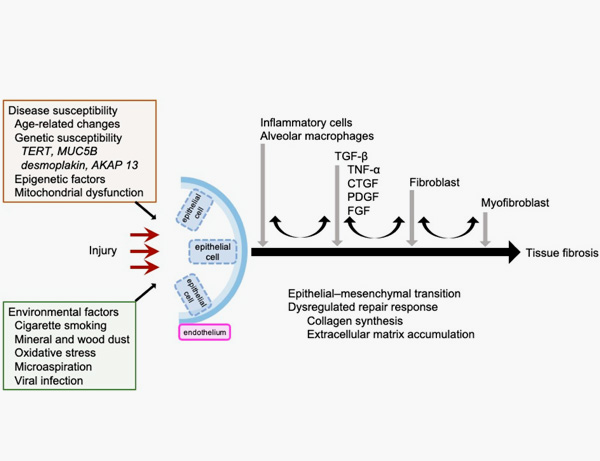
9. Inui N, Sakai S, Kitagawa M. Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway. Int J Mol Sci. 2021 Jun 5;22(11):6107.
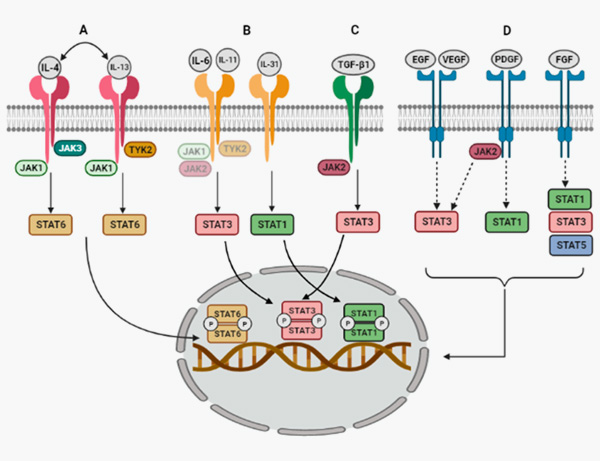
10. Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211.
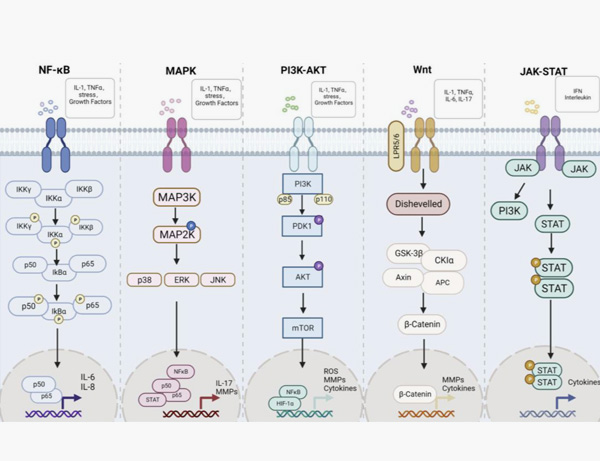
11. Zhu, M.; Ding, Q.; Lin, Z.; Fu, R.; Zhang, F.; Li, Z.; Zhang, M.; Zhu, Y. New Targets and Strategies for Rheumatoid Arthritis: From Signal Transduction to Epigenetic Aspect. Biomolecules 2023, 13, 766.
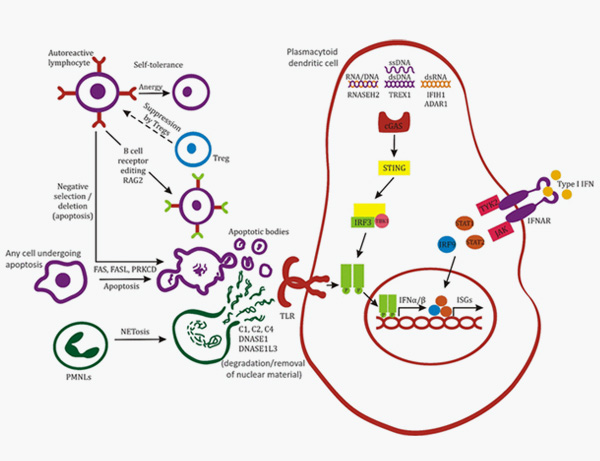
12. Batu, Ezgi. (2018). Monogenic systemic lupus erythematosus: insights in pathophysiology. Rheumatology International. 38. 10.1007/s00296-018-4048-7.
This web search service is supported by Google Inc.