
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Tools for ADC PK Analysis——Anti-payload antibodies | Type | Analyte Detail | Typical Method |
|---|---|---|
| Total Antibody | Conjugated, partially unconjugated, and fully unconjugated (DAR > 0) | Ligand Binding Assay |
| Conjugated Antibody | Antibody with minimum DAR > 1 | Ligand Binding Assay |
| Antibody-conjugated Drug | Total small-molecule drug conjugated to Antibody | Affinity LC-MS / Ligand Binding Assay |
| Unconjugated Drug | Small molecule drug not conjugated to antibody | LC-MS |
| Total Drug | Total unconjugated and conjugated Drug | LC-MS |
ADC molecule type and typical analytical methods for evaluation. 1
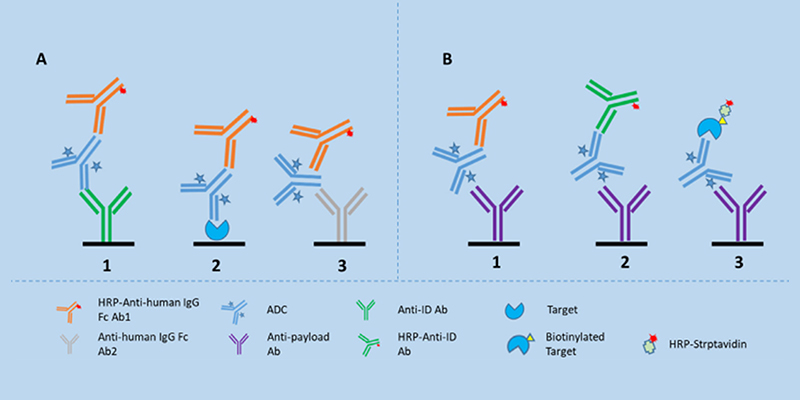
Different LBA formats to analyze ADCs in serum 2
| Molecule | Cat. No. | Product Description | Preorder/Order |
|---|
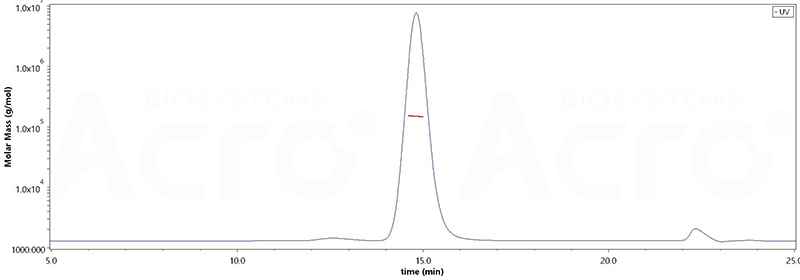
The purity of Mouse Anti-MMAE Antibody, Mouse IgG1 (Cat. No. MME-M5252) is more than 90% and the molecular weight of this protein is around 134-163 kDa verified by SEC-MALS.
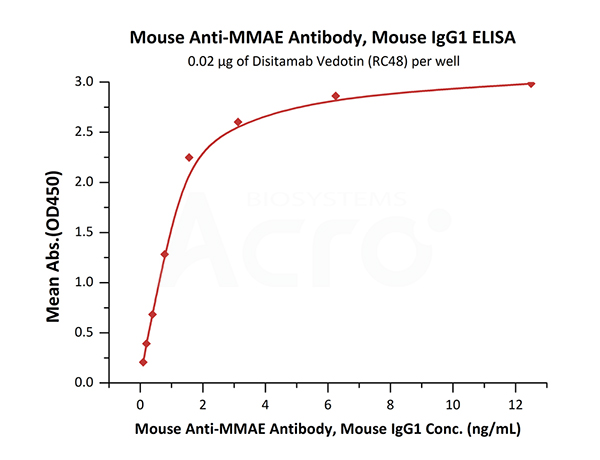
Immobilized Disitamab Vedotin (RC48) at 0.2 μg/mL (100 μL/well) can bind Mouse Anti-MMAE Antibody, Mouse IgG1 (Cat. No. MME-M5252) with a linear range of 0.1-2 ng/mL (QC tested).
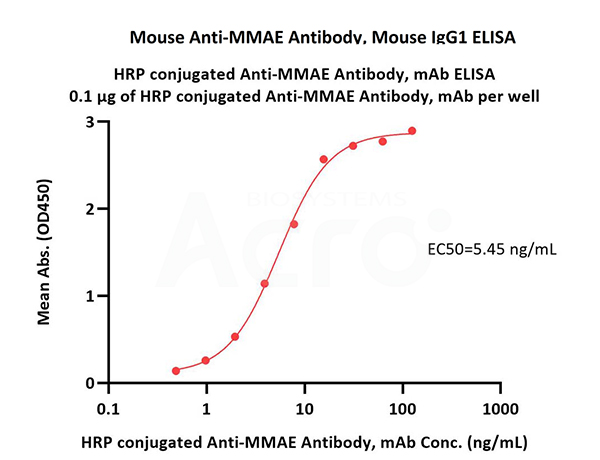
Immobilized Disitamab Vedotin at 1 μg/mL (100 μL/well) can bind HRP conjugated Anti-MMAE Antibody, mAb (Cat. No. MME-PLS104) with a linear range of 0.5-16 ng/mL (QC tested).
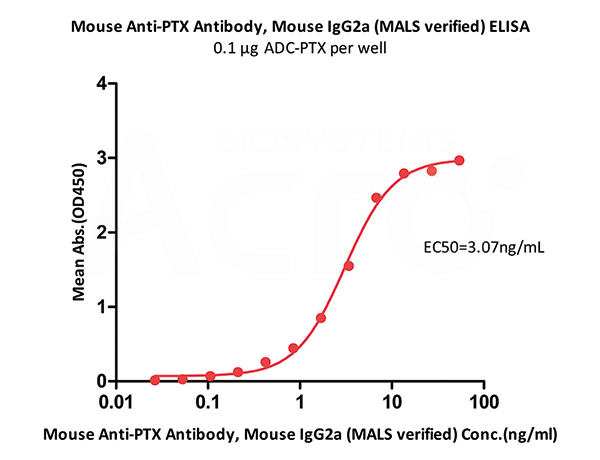
Immobilized ADC-PTX at 1 μg/mL (100 μL/well) can bind Mouse Anti-PTX Antibody, Mouse IgG2a (MALS verified) (Cat. No. PTX-S343) with a linear range of 0.106-6.767 ng/mL (QC tested).
| Molecule | Cat. No. | Product Description | Neutralizing activity | Application |
|---|---|---|---|---|
| Adalimu*ab | ADB-Y19 | Anti-Adalimu*ab Antibodies (AY19) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Adalimu*ab | ADB-Y23b | Anti-Adalimu*ab Antibodies (AY23b) (recommended for PK/PD) | Non-Neutralizing Antibody | PK bridging ELISA; Indirect ELISA |
| Bevacizu*ab | BEB-Y10 | Anti-Bevacizu*ab Antibodies (AY10) (MALS verified, recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y12 | Anti-Bevacizu*ab Antibodies (AY12) (recommended for neutralizing assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y9 | Anti-Bevacizu*ab Antibodies (AY9) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-BY13 | Biotinylated Anti-Bevacizu*ab Antibodies (AY13) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y27 | Anti-Cetuxi*ab Antibodies (AY27) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y31 | Anti-Cetuxi*ab Antibodies (AY31) (Non-Neutralizing) | Non-Neutralizing Antibody | ADA assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y28 | Anti-Cetuxi*ab Antibodies (AY28) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-BY31 | Biotinylated Anti-Cetuxi*ab Antibodies (AY31) (recommended for PK/PD) | Non-Neutralizing Antibody | PK bridging ELISA; Indirect ELISA |
| Rituxi*ab | RIB-Y36 | Anti-Rituxi*ab Antibodies (AY36) (recommended for ADA assay) | Neutralizing Antibody | ADA assay;Neutralizing assay; Indirect ELISA |
| Rituxi*ab | RIB-Y37 | Anti-Rituxi*ab Antibodies (AY37) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay;Indirect ELISA |
| Rituxi*ab | RIB-Y35c | Anti-Rituxi*ab Antibodies (MALS verified) | Non-Neutralizing Antibody | ADA assay; Indirect ELISA |
| Trastuzu*ab | TRB-Y5b | Anti-Trastuzu*ab Antibodies (AY5b) (recommended for PK/PD) | Non-Neutralizing Antibody | Neutralizing Antibody |
| Trastuzu*ab | TRB-Y1b | Anti-Trastuzu*ab Antibodies (AY1b) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA; Neutralizing assay; Indirect ELISA |
>> Click to learn more core reagents accelerating ADC’s development!

This web search service is supported by Google Inc.










