 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
Various proteins on the cell membrane are responsible for cell protection, internal/external material transportation, and signal transmission. These membrane proteins play diverse roles in cell biology and have become a crucial target in drug development. Over 50% of the currently approved drugs target human membrane proteins. G protein-coupled receptors (GPCRs) are the largest family of membrane proteins. The primary function of GPCRs is to transduce a wide and diverse array of extracellular stimuli such as biogenic amines, peptides, hormones, neurotransmitters, ions, odorants, and photons into intracellular signals. This signaling, in turn, regulates a myriad of physiological processes, including cell metabolism, differentiation, growth, neurotransmission, and sensory perception. GPCRs are implicated in various diseases, including type 2 diabetes mellitus (T2DM), obesity, depression, HIV, cancer, Alzheimer's disease, etc. The crucial role of GPCRs and the increased efforts in the drug discovery field have led to GPCRs becoming the most successful drug target class in the treatment of various pathologies. This review discusses the structure of GPCRs, signaling pathways, and the development pattern of various stages of targeted drugs. ACROBiosystems is focused on overcoming the challenges of GPCRs preparation and providing full-length GPCRs proteins to assist our customers and collaborating researching and developing antibody-drug and therapy strategies.
In this manuscript, a comprehensive review of GPCR structure, function, expression tips, and products developed by ACROBiosystems will be summarized and reviewed for reference.
The common molecular structure of GPCRs consists of seven transmembrane alpha helices, and these domains divide the receptor into extracellular N-terminus, intracellular C-terminus, three extracellular loops and three intracellular loops. The extracellular ring contains two highly conserved cysteine residues, which can stabilize the spatial structure of the receptor by forming disulfide bonds. There is a G-protein binding site on the intracellular loop. In the case of CCR5, the classical GPCRs topology is shown in Figure 1 below (1).
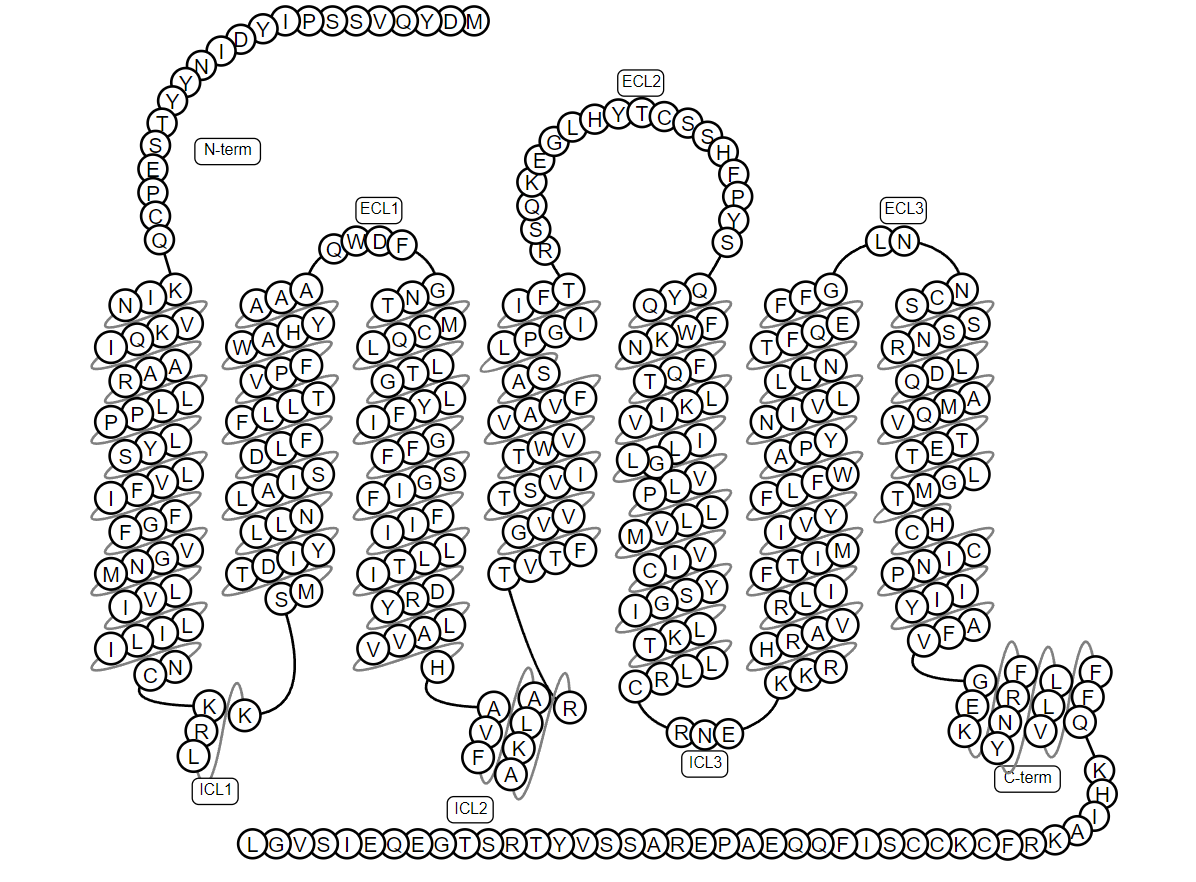
Figure 1. Schematic diagram of the classic GPCRs (1)
The effectors of GPCR activation are the heterotrimeric G-proteins Gα, Gβ, and Gγ. Activated GPCRs act as GEFs (guanine-nucleotide-exchange factors) and exchange GDP for GTP in the Gα subunit, which activates the protein. A GPCR is free of ligand (L) in its basal state. Gα binds to GDP and is associated with Gβγ. The heterotrimeric protein complex might associate with the receptor at this point or remain free in the membrane as pictured, but once it encounters a ligand-bound GPCR, downstream signaling is initiated (Figure 2). Upon ligand binding, the GPCR becomes activated and undergoes a conformational change. The resulting GTP-bound Gα separates from βγ and the active heterotrimeric proteins. Currently, the GDP-αβγ complex, with the participation of Mg2+, exchanges GTP in the bound GDP with the cytosol to form a GTP-αβγ complex. Then the G protein is activated and separated from the receptor, and simultaneously disassemble into two parts, GTP-α and βγ, which diffuse freely along the cell membrane and directly interact with effector proteins, such as PLC or adenylate cyclase, which results in effector activation and initiation of a second-messenger cascade and complete the extracellular transmission of signals into the cell. The GTP in Gα is then hydrolyzed to GDP through the activity of Gα and RGS proteins (not shown), leading to Gα inactivation and reassociation of the heterotrimeric protein complex. This process represents a full GPCR G-protein cycle.
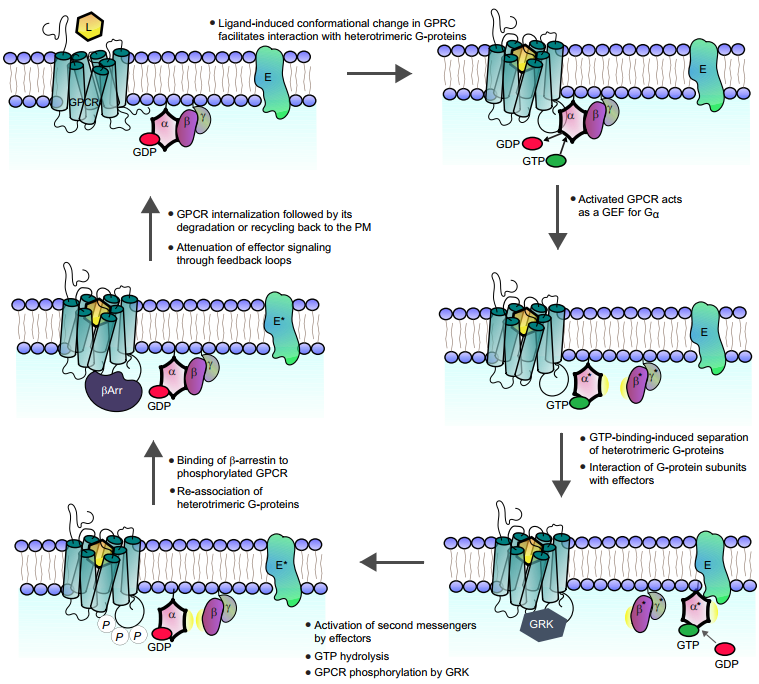
Figure 2. GPCRs signal pathway (2)
GPCRs are the most intensively studied drug targets comprising approximately 27% of the global market share of therapeutic drugs, with aggregated sales for 2011-2015 of $890 billion. There are 227 (57%) non-olfactory GPCRs that are yet to be studied in a clinical setting but have broad untapped therapeutic potential, particularly in genetic and immune system disorders.
In 2017, survey data showed 481 therapeutic drugs targeting GPCRs, which compose 34% of FDA-approved drugs and mediate their effects through at least 107 unique GPCRs. Approximately 320 drugs are currently in clinical trials, of which 35% of these drugs 64 potential novel GPCRs targets (3).
In the field of drug development that targets GPCRs, GPCRs antibodies have unique advantages over small molecule drugs:
The clearance rate in the body is lower, action time is longer, and the corresponding administration frequency is lower.
The selectivity of antibodies is significantly higher than that of small molecules.
However, due to the barrier of the blood-brain barrier, antibody drugs cannot enter the central nervous system. Therefore, for GPCRs that are expressed in both the peripheral and central nervous systems, only the peripheral part of the drug needs to be designed, therapeutic antibodies can be developed to make the drug mainly distributed in the peripheral area and reduce the toxic side effects on the central nervous system.
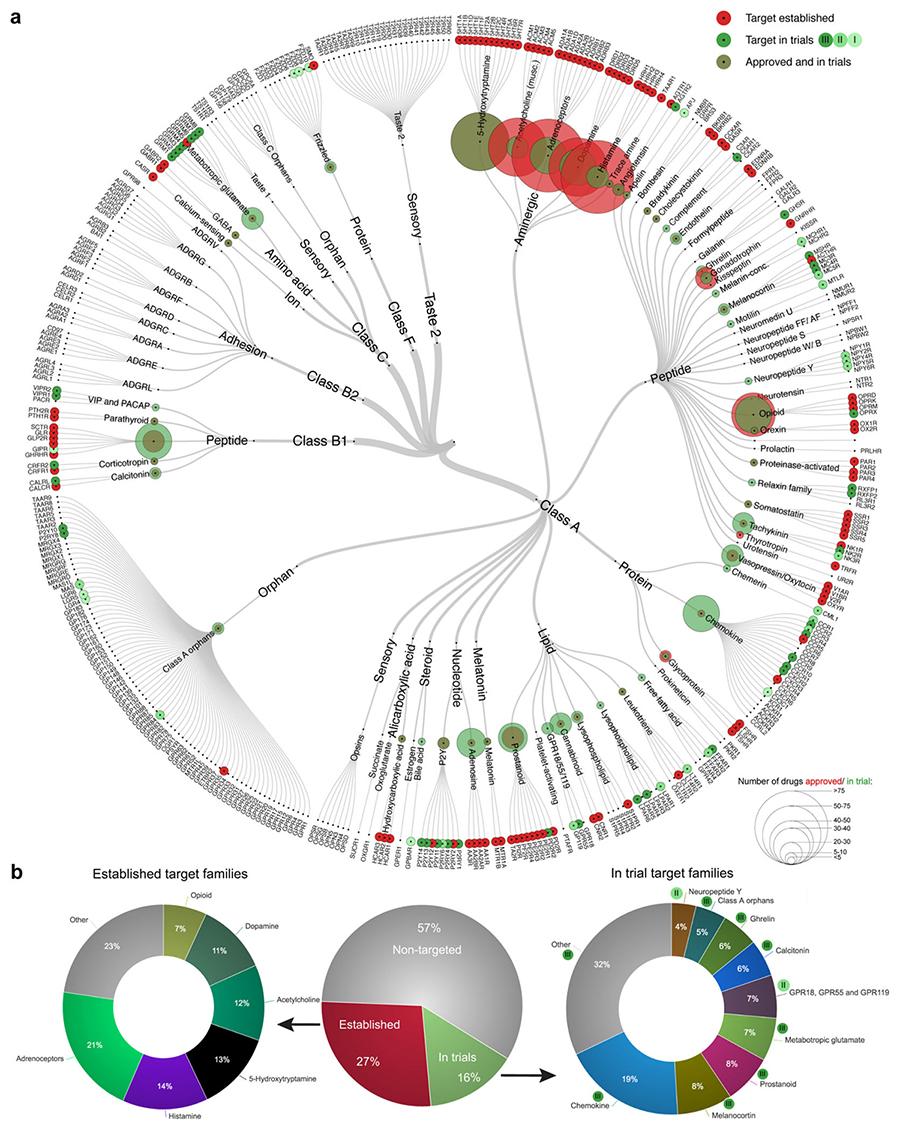
Figure 3. GPCRs clinical drug target distribution (3)
There are currently about 13 GPCRs targeted antibody projects under development that are active worldwide, as shown in the table below. The target is mainly concentrated in CCR4, CALCRL, CCR5, GCGR, GPRC5D, GLP1R, C5AR, CB1, S1PR1, CCR8, CCR7, GPR49, AGTR1. The representative drug is Leronlimab, a humanized IgG4 monoclonal antibody targeting CCR5, an HIV viral entry inhibitor. By masking CCR5, the HIV (R5) subtype is blocked from entering healthy T cells, thereby protecting these cells from viral infections.
Targeted GPCRs drug information under research (CLARIVATE Database)
| Drug Name | Target | Organization | Highest Phase | Condition |
|---|---|---|---|---|
| Mogamulizumab | CCR4 | Kyowa Hakko Kirin | Launched | Lymphoma Therapy |
| Eptinezumab | CGRPR | Alder Biopharmaceuticals | Launched | Acute Attacks of Migraine |
| Leronlimab | CCR5 | Beth Israel Deaconess Medical Center | Pre-Registered | Anti-HIV Agents, Cancer |
| GMA102 | GLP1R | Gmax Biopharm | Phase III | Type 2 Diabetes |
| Ulocuplumab | CXCR4 | Bristol-Myers Squibb | Phase II | Small cell lung cancer, Pancreatic cancer, Multiple myeloma, Leukemia |
| Volagidemab | GCGR | Amgen | Phase II | Type 2 Diabetes, |
| Talquetamab | CD3, GPRC5D | Janssen | Phase II | Multiple Myeloma Therapy |
| Avdoralimab | C5AR | Novo Nordisk | Phase II | COVID-19, solid tumors |
| Nimacimab | CB1 | Bird Rock Bio | Phase II | Diabetic Nephropathy |
| Sonepcizumab | S1PR1 | Pfizer & Merck Serono | Phase II | Age-Related Macular Degeneration |
| BMS-986340 | CCR8 | Bristol-Myers Squibb | Phase I/II | Solid Tumor Treatments |
| MCLA-158 | EGFR, LGR5 | Merus | Phase I | Colorectal Cancer Therapy |
The first step in preparing antibodies is antigen immunity, and the first step in immunity is the preparation of antigen. Since GPCRs are seven-pass transmembrane proteins, it is extremely difficult to obtain biologically active soluble GPCRs antigen.
To obtain a full-length GPCRs antigen for drug development, you need to break through two bottlenecks:
How to increase the expression? The expression of most membrane proteins on the cell membrane is limited, and some are even expressed only in a short period during a specific period of the cell cycle. In addition, unlike secretory proteins, the expression and display of membrane proteins are limited by the membrane area. Many membrane proteins involve functions related to material transport and signaling; overexpression on the cell membrane can cause irreversible damage to cells. These characteristics of membrane proteins greatly limit their expression. Therefore, to obtain enough full-length membrane proteins for immunity and drug development, it is necessary to design and optimize the expression interval, expression system, culture conditions, etc. Even so, the cost of obtaining milligram membrane proteins is still much higher than that of soluble proteins.
How to maintain the uniformity and activity of membrane proteins during expression and purification? The transmembrane domain of membrane proteins is highly hydrophobic, so unprotected exposure to water will lead to nonspecific protein aggregation and even denaturation. Therefore, in the process of enrichment and purification of membrane proteins, it is necessary always to maintain the hydrophilic properties of the surrounding environment of membrane proteins. The most widely used method involves removing membrane proteins from the cell membrane of the phospholipid bilayer through detergent (4) and forming micelles to maintain their natural conformation and functional activity as much as possible. Furthermore, there are also methods such as Nanodisc (5), virus-like particles (VLP) (6), polymer lipid particles (PoLiPa) (7).
Therefore, to meet different application needs in the drug development process that targets GPCRs, ACROBiosystems has specially set up platform solutions with VLP, Detergent, and Nanodisc to provide full-length GPCRs proteins such as GPRC5D, CXCR4, CCR5, CCR8, etc.
GPCRs related products available from ACROBiosystems
| Platform | Molecule | Cat. No. | Product Description |
|---|---|---|---|
| VLP Platform | GPRC5D | GPD-H52P7 | Human GPRC5D Full Length Protein-VLP (HEK293) |
| GPRC5D | GPD-HF2G5 | Fluorescent Human GPRC5D Full Length Protein-VLP (HEK293) | |
| CCR5 | CC5-H52P3 | Human CCR5 Full Length Protein-VLP (HEK293) | |
| CCR8 | CC8-H52P4 | Human CCR8 Full Length Protein-VLP (HEK293) | |
| CXCR4 | CX4-H5219 | Human CXCR4 / CD184 Full Length Protein-VLP (HEK293) | |
| VLP | VLP-N5213 | Virus-Like Particle (VLP) isotype control | |
| Micelle Detergent Platform | GPRC5D | GPD-H52D3 | Human GPRC5D Protein, Flag,His Tag |
| GPRC5D | GPD-H82D6 | Biotinylated Human GPRC5D Protein, His,Avitag™&Flag Tag | |
| GPRC5D | GPD-C52D3 | Cynomolgus GPRC5D Protein, Flag,His Tag | |
| CCR5 | CC5-H52D1 | Human CCR5 Protein, Flag,His Tag | |
| Buffer | DC-11 | 200 x DDM CHS buffer | |
| Nanodisc Platform | GPRC5D | GPD-H52D4 | Human GPRC5D Protein, Flag,His Tag (Nanodisc) |
| GPRC5D | GPD-H82D4 | Biotinylated Human GPRC5D Protein, His,Avitag™&Flag Tag (Nanodisc) | |
| GPRC5D | GPD-C82D4 | Cynomolgus GPRC5D Protein, Flag,His Tag (Nanodisc) | |
| MSP1D1 | APO-H51H3 | Human MSP1D1 Protein, His Tag (Nanodisc) | |
| MSP1D1 | APO-H81Q5 | Biotinylated Human MSP1D1 Protein, His,Avitag™ (Nanodisc) |
In addition to GPCRs, ACROBiosystems can provide a complete range of full-length multi-pass transmembrane proteins, including four-pass transmembrane protein CD20, Claudin18.2, and five-pass transmembrane protein CD133. More full-length proteins are under development, so stay tuned and consult.
1. Dong HF, Wigmore K, Carrington MN, Dean M, Turpin JA, Howard OM. Variants of CCR5, which are permissive for HIV-1 infection, show distinct functional responses to CCL3, CCL4 and CCL5. Genes Immun. 2005 Oct;6(7):609-19. doi: 10.1038/sj.gene.6364247. PMID: 16015368; PMCID: PMC1369982.
2. Hanlon CD, Andrew DJ. Outside-in signaling--a brief review of GPCR signaling with a focus on the Drosophila GPCR family. J Cell Sci. 2015 Oct 1;128(19):3533-42. doi: 10.1242/jcs.175158. Epub 2015 Sep 7. PMID: 26345366; PMCID: PMC4610211.
3. Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub 2017 Oct 27. PMID: 29075003; PMCID: PMC6882681.
4. Wiseman DN, Otchere A, Patel JH, Uddin R, Pollock NL, Routledge SJ, Rothnie AJ, Slack C, Poyner DR, Bill RM, Goddard AD. Expression and purification of recombinant G protein-coupled receptors: A review. Protein Expr Purif. 2020 Mar;167:105524. doi: 10.1016/j.pep.2019.105524. Epub 2019 Oct 31. PMID: 31678667; PMCID: PMC6983937.
5. McLean MA, Gregory MC, Sligar SG. Nanodiscs: A Controlled Bilayer Surface for the Study of Membrane Proteins. Annu Rev Biophys. 2018 May 20;47:107-124. doi: 10.1146/annurev-biophys-070816-033620. Epub 2018 Mar 1. PMID: 29494254; PMCID: PMC6370528.
6. Ho TT, Nguyen JT, Liu J, Stanczak P, Thompson AA, Yan YG, Chen J, Allerston CK, Dillard CL, Xu H, Shoger NJ, Cameron JS, Massari ME, Aertgeerts K. Method for rapid optimization of recombinant GPCR protein expression and stability using virus-like particles. Protein Expr Purif. 2017 May;133:41-49. doi: 10.1016/j.pep.2017.03.002. Epub 2017 Mar 3. PMID: 28263854.
7. Hothersall JD, Jones AY, Dafforn TR, Perrior T, Chapman KL. Releasing the technical 'shackles' on GPCR drug discovery: opportunities enabled by detergent-free polymer lipid particle (PoLiPa) purification. Drug Discov Today. 2020 Aug 21:S1359-6446(20)30337-8. doi: 10.1016/j.drudis.2020.08.006. Epub ahead of print. PMID: 32835806.
This web search service is supported by Google Inc.
