 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
For a long time, IgG has been the only class of antibodies that are actively transferred from mother to offspring. This results in short-term passive immunity, and the specific IgG transport is accomplished by FcRn. In 1972, Jones et.al. first identified a receptor that transport maternal IgG to newborns in the gut of neonatal rats, hence after it was named as Neonatal Fc receptor (FcRn). FcRn expression during pregnancy and lactation plays a role in transporting IgG across the placental barrier and the intestinal tract. A variety of tissue cells can be detected throughout the life cycle. The main function is to maintain the level of IgG and albumin in the serum and regulate the distribution in the tissue.
IgG has a half-life of up to 2 to 4 weeks in the human body. The impact of the half-life is mainly due to the FcRn-mediated recycling mechanism. This mechanism is achieved by the pH-dependent binding of IgG Fc fragment and serum albumin to FcRn.
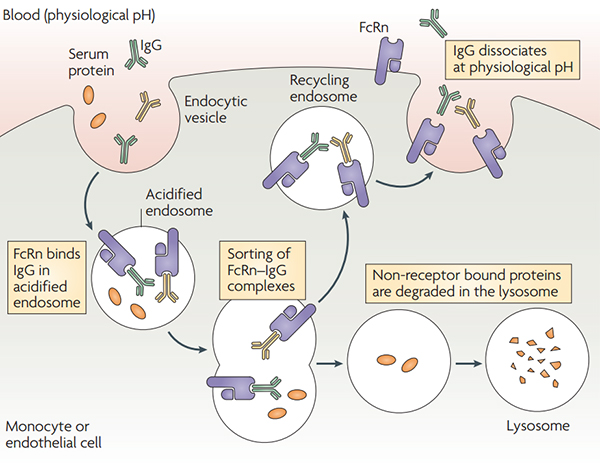
Under acidic conditions (pH6.0-6.5), FcRn binds IgG, and dissociation occurs under neutral and weakly alkaline conditions (pH7.0-7.5). Specifically, endothelial cells form endocytosis vesicles by ingesting IgG to form an acidified endosome, and IgG binds to FcRn to form an IgG-FcRn complex. The IgG-FcRn complex is transported to the cell surface through the recirculated endosome. Under physiological conditions such as pH7.4, the IgG-FcRn complex is dissociates, and the IgG is released again into the blood circulation. Through this receptor-mediated recycling mechanism, FcRn effectively protects IgG from lysosomal degradation, thus prolonging the half-life of IgG. However, IgG does not bind to FcRn to form an IgG-FcRn complex in the acidified body, degrades in the lysosome. Studies have shown that the FcRn-mediated IgG recovery rate is 42% higher than the IgG production rate, suggesting that IgG recycling is the main process that maintains IgG concentrations in human body.
FcRn is a heterodimer composed of two subunits FCGRT and B2M. FCGRT has three extracellular functional regions including three soluble domains (α1, α2, and α3), a single transmembrane helix and a cytoplasmic tail (some studies showed the cytoplasmic tail region composed of 44 amino acid residues that may contain signals that mediate intracellular pathways). Its molecular weight is 40 to 50 kDa, called the α chain while the molecular weight of B2M is 14 kDa, called the β chain. The two chains are joining in the form of non-covalent bonds. FCGRT must be assembled with B2M to play a transport role. Studies have shown that the binding site of FcRn with IgG and serum albumin is not the same, so the binding of FcRn to IgG is not interfered by serum protein.
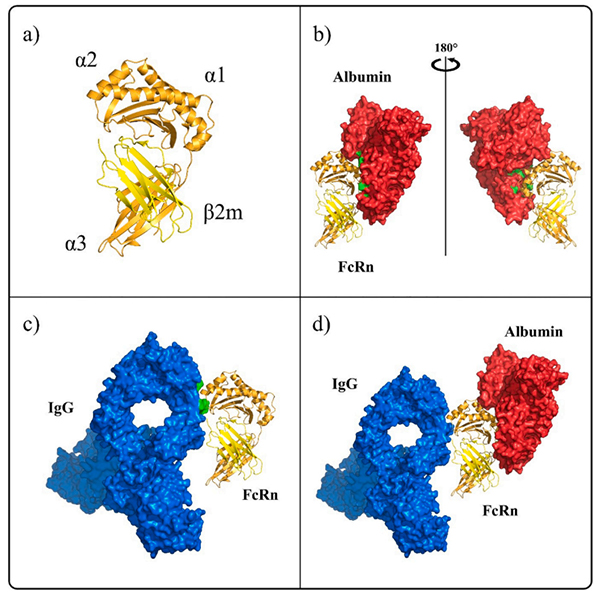
FcRn has different binding sites with ligand IgG and albumin
The high affinity of FcRn has harmful effects on IgG-mediated autoimmune diseases such as myasthenia gravis, rheumatoid arthritis, or pemphigus vulgaris. Targeting FcRn and inhibiting FcRn circulation can enhance IgG catabolism, resulting in overall reduced IgG and pathogenic autoantibody levels, which is expected to reduce all autoimmune abnormalities caused by IgG. Studies have confirmed that FcRn targeting therapy provides faster and more selective IgG reduction than therapeutic plasma exchange therapy for myasthenia gravis. In addition, the advantages of this treatment over many clinically use conventional therapies are also demonstrated by the fact that IgA, IgD, IgE, and IgM are not dependent on FcRn-mediated circulation. Therefore, it does not lead to extensive immunosuppression, providing the basis for the safety and precision specificity of FcRn targeting.
Drugs targeting FcRn antibodies currently being evaluated in clinical trials include Orilanolimab (SYNT001), Efgartigimo, Nipocalimab (M281) and so on. Nipocalimab (M281) is a potential “best-in-class”anti-FcRn antibody developed by Momenta. It has the potential to treat a variety of autoimmune diseases and was introduced by Johnson & Johnson. Batoclimab is a Hebo Pharmaceutical, introduced from HanAll Biopharma in 2018. It is also a whole human antibody targeting the Fc receptor (FcRn) of newborns. They have obtained the rights to develop, produce and commercialize it in the Greater China region.
Global Clinical Trials of FcRn Targeting for Autoimmune Diseases
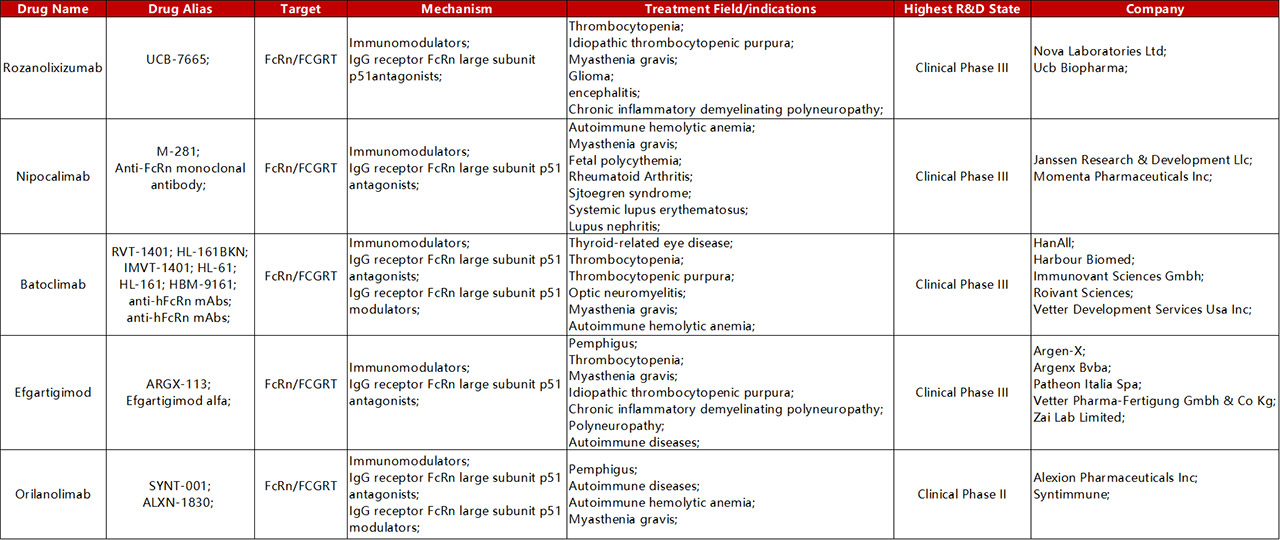
Drugs that target FcRn do not use FcRn as a direct target for therapeutic intervention as conventional targets. Instead, it uses the advantages of cellular drug delivery, which is called a "revolutionary therapy" that can wipe out all autoimmune diseases, with high potential. Focusing on the potential of targeting FcRn in autoimmune diseases, major pharmaceutical companies have poured into the track, promising to treat all autoimmune diseases caused by autoantigens.
To support monoclonal antibody, bispecific antibody, ADC drug affinity research and targeted FcRn drug development for the treatment of autoimmune diseases, ACROBiosystems can provide you with 19 high-quality FcRn protein products.
 Expressed by HEK293 Cells: post-translational modification and proper protein folding
Expressed by HEK293 Cells: post-translational modification and proper protein folding
 Multiple species: Human, Mouse, Cynomolgus/Rhesus macaque, Rat, Porcine, Rabbit, Feline, Bovine, can be fully applied to different cross species experiments
Multiple species: Human, Mouse, Cynomolgus/Rhesus macaque, Rat, Porcine, Rabbit, Feline, Bovine, can be fully applied to different cross species experiments
 High purity: SDS-PAGE verification purity>95%, SEC-MALS verification purity>90%
High purity: SDS-PAGE verification purity>95%, SEC-MALS verification purity>90%
 Low endotoxin: <1.0 EU/µg
Low endotoxin: <1.0 EU/µg
 High stability: strict quality control to ensure high batch-to-batch consistency
High stability: strict quality control to ensure high batch-to-batch consistency
 Biotinylated FcRn proteins labeled with AvitagTM offered . The labeling efficiency is high, and the labeling site is specific and clear, which is suitable for ELISA/SPR/BLI detection based on binding to streptavidin in the process of drug development and optimization process
Biotinylated FcRn proteins labeled with AvitagTM offered . The labeling efficiency is high, and the labeling site is specific and clear, which is suitable for ELISA/SPR/BLI detection based on binding to streptavidin in the process of drug development and optimization process
 Affinity verified by SPR & BLI: activity guaranteed, and protocols offered free of charge
Affinity verified by SPR & BLI: activity guaranteed, and protocols offered free of charge
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|---|---|---|---|
| FcRn (FCGRT & B2M) | FCM-H5286 | HEK293 | Human FcRn / FCGRT&B2M Heterodimer Protein, His Tag&Strep II Tag (SPR & BLI & MALS verified)Hot |  |
| FCM-H8286 | HEK293 | Biotinylated Human FcRn / FCGRT&B2M Heterodimer Protein, His Tag&Strep II Tag, ultra sensitivity (primary amine labeling) (SPR verified) |  | |
| FCM-H82W4 | HEK293 | Biotinylated Human FcRn / FCGRT&B2M Heterodimer Protein,,His Tag&Strep II Tag (SPR & BLI & MALS verified)Hot | 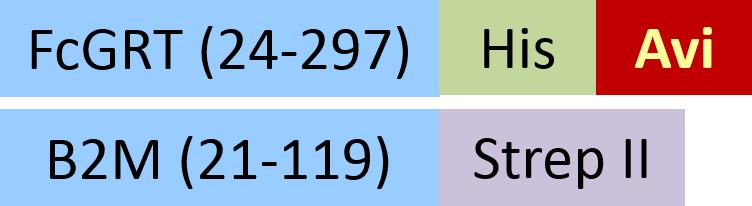 | |
| FCM-H82W7 | HEK293 | Biotinylated Human FcRn / FCGRT&B2M Heterodimer Protein, His,(MALS & SPR verified) |  | |
| FCN-H52W7 | HEK293 | Human FcRn / FCGRT&B2M Heterodimer Protein, His Tag (SPR & BLI & MALS verified)Hot |  | |
| FCM-M52W2 | HEK293 | Mouse FcRn / FCGRT&B2M Heterodimer Protein, His Tag&Twin-Strep Tag (MALS & BLI verified) |  | |
| FCM-M52W8 | HEK293 | Mouse FcRn / FCGRT&B2M Heterodimer Protein, His Tag (MALS & BLI verified) |  | |
| FCM-M82W5 | HEK293 | Biotinylated Mouse FcRn / FCGRT&B2M Heterodimer Protein, His,(MALS & BLI verified) |  | |
| FCM-M82W6 | HEK293 | Biotinylated Mouse FcRn / FCGRT&B2M Heterodimer Protein,,His Tag&Twin-Strep Tag (MALS & BLI-verified) |  | |
| FCM-R5287 | HEK293 | Rat FcRn / FCGRT&B2M Heterodimer Protein, His Tag&Strep II Tag (MALS & SPR verified) | 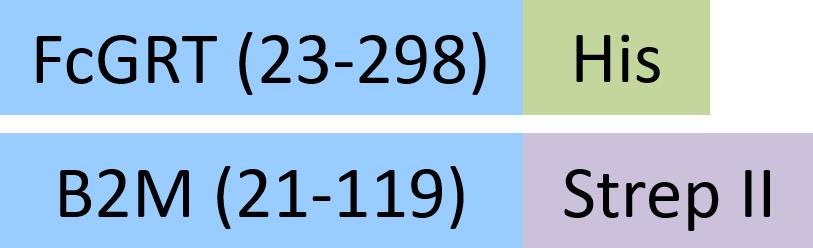 | |
| FCM-R82W7 | HEK293 | Biotinylated Rat FcRn / FCGRT&B2M Heterodimer Protein,,His Tag&Strep II Tag (MALS & SPR verified) |  | |
| FCM-R52W5 | HEK293 | Rabbit FcRn / FCGRT&B2M Heterodimer Protein, His Tag&Tag Free (MALS & SPR verified) |  | |
| FCM-C5284 | HEK293 | Cynomolgus / Rhesus macaque FcRn / FCGRT&B2M Heterodimer Protein, His Tag&Strep II Tag (MALS & BLI verified) |  | |
| FCM-C52W9 | HEK293 | Cynomolgus / Rhesus macaque FcRn / FCGRT&B2M Heterodimer Protein, His Tag (MALS & BLI verified) |  | |
| FCM-C82W3 | HEK293 | Biotinylated Cynomolgus / Rhesus macaque FcRn / FCGRT&B2M Heterodimer Protein, His,(MALS & SPR verified) |  | |
| FCM-C82W5 | HEK293 | Biotinylated Cynomolgus / Rhesus macaque FcRn Heterodimer Protein, His,&Strep II Tag (SPR & BLI verified) | 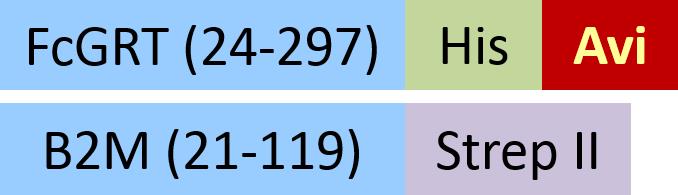 | |
| FCM-P5280 | HEK293 | Porcine FcRn / FCGRT&B2M Heterodimer Protein, His Tag&Strep II Tag (MALS & SPR verified) |  | |
| FCN-F82W3 | HEK293 | Biotinylated Feline FcRn / FCGRT&B2M Heterodimer Protein, His,(MALS & SPR verified) |  | |
| FCN-B82W3 | HEK293 | Biotinylated Bovine FcRn / FCGRT&B2M Heterodimer Protein, His,(SPR verified) |  |
>>> Click to view high-quality FcRn proteins and download free protocols


References
[1] Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. The Journal of Clinical Inveatigation. 1972, 51:2916–27.
[2] Derry C. Roopenian, Shreeram Akilesh. FcRn: the neonatal Fc receptor comes of age. Nature Reviews Immunology. 2007,7:715–725.
[3] Imke Rudnik-Jansen, Kenneth A. Howard. FcRn expression in cancer: Mechanistic basis and therapeutic opportunities. Journal of Controlled Release. 2021, 337 :248–257.
[4] Karissa L. Gable, Jeffrey T. Guptill. Antagonism of the Neonatal Fc Receptor as an Emerging Treatment for Myasthenia Gravis. Frontiers in Immunology. 2020, 10.
This web search service is supported by Google Inc.
