 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
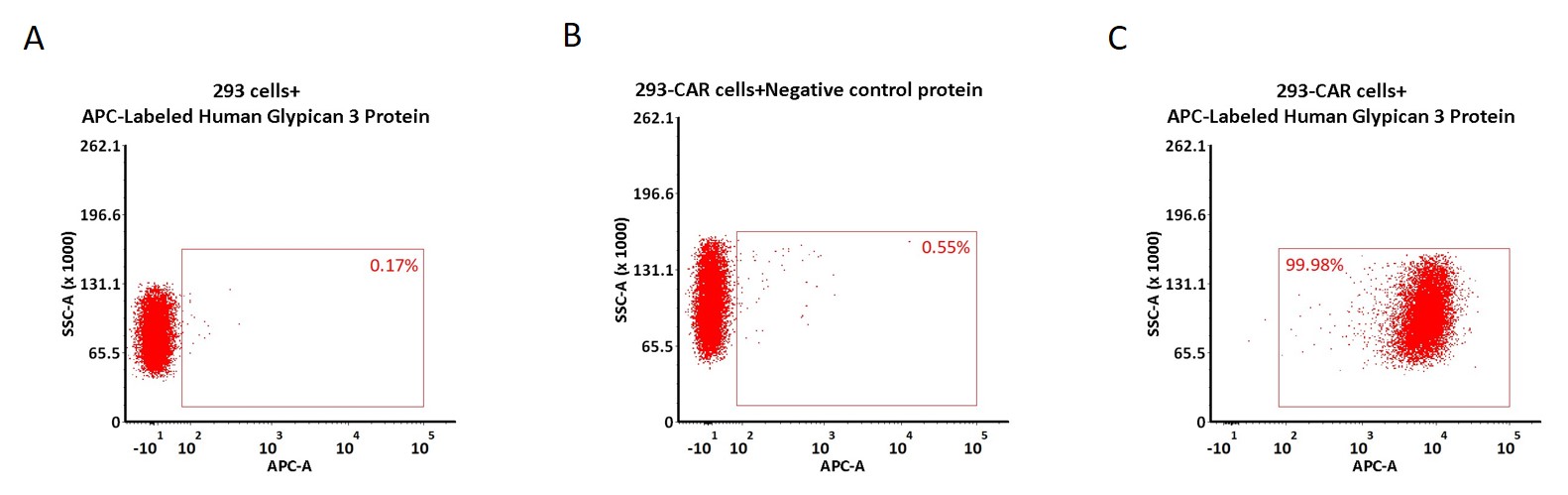
5e5 of anti-GPC3 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of APC-Labeled Human Glypican 3 Protein, His Tag (Cat. No. GP3-HA2H7) and negative control protein respectively (Fig. C and B), and non-transfected 293 cells were used as a control (Fig. A). APC signal was used to evaluate the binding activity (QC tested).
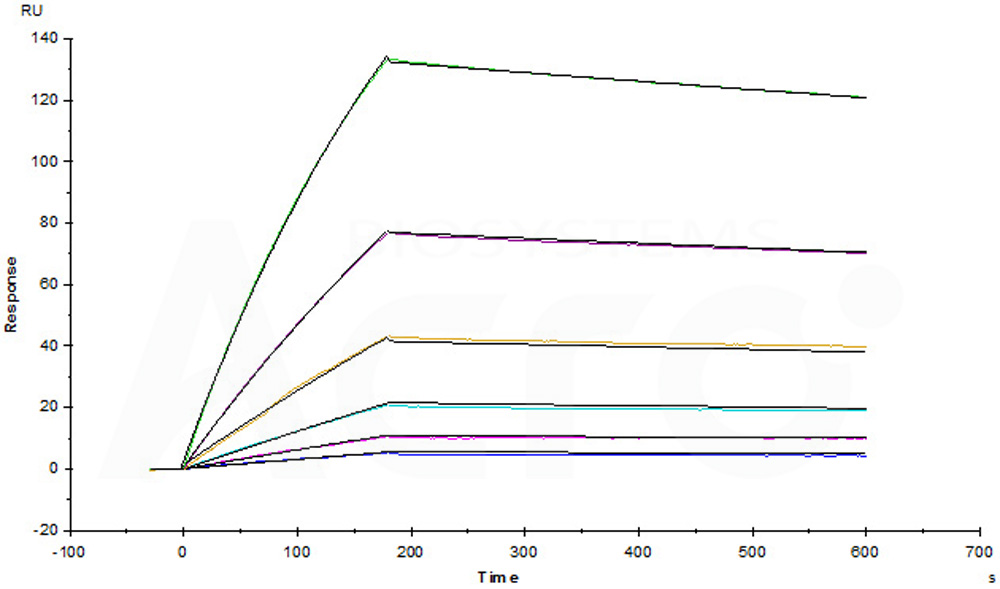
Anti-human GPC3 MAb (chimeric mouse-human IgG1) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human Glypican 3, His Tag, premium grade (Cat. No. GP3-H52H4) with an affinity constant of 1.33 nM as determined in a SPR assay (Biacore T200) (Routinely tested).

Anti-Human GPC3 MAb (chimeric mouse-human IgG1) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human Glypican 3 (S359F) Protein, His Tag (Cat. No. GP3-H5223) with an affinity constant of 1.96 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ERY-974 | ERY-974 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| ECT-204 | ECT-204; JWATM-204 | Phase 2 Clinical | Eureka Therapeutics Inc | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| GPC3-CAR-T cell therapy(Origincell Medical Technology) | Ori-CAR-001 | Phase 1 Clinical | Carcinoma, Hepatocellular | Details | |
| Anti-GPC3-IRDye800CW | Clinical | Chinese Academy Of Sciences | Details | ||
| CM-350 | CM-350 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Solid tumours | Details |
| IM-83 CAR-T cell therapy | IM-83 | Phase 1 Clinical | Beijing Immunochina Medical Science & Technology Co Ltd | Liver Neoplasms | Details |
| TC-CAR-031 | TC-CAR-031; TC-CAR031 | Phase 1 Clinical | Zhejiang University | Carcinoma, Hepatocellular | Details |
| GPC3 CAR-T therapy (Hrain Biotechnology) | Phase 2 Clinical | Hrain Biotechnology Co Ltd | Carcinoma, Hepatocellular | Details | |
| CAR-GPC3 T-cell (Drum Tower Hospital) | Phase 2 Clinical | Nanjing Drum Tower Hospital | Carcinoma, Hepatocellular | Details | |
| Anti-GPC3 CAR T-cell therapy (Nanjing University) | Phase 1 Clinical | Nanjing University | Carcinoma, Hepatocellular | Details | |
| GLYCAR T cell therapy (Baylor College of Medicine) | GLYCAR | Phase 1 Clinical | Baylor College Of Medicine | Liver Neoplasms; Rhabdomyosarcoma; Rhabdoid Tumor; Hepatoblastoma; Wilms Tumor; Liposarcoma; Endodermal Sinus Tumor; Carcinoma, Hepatocellular | Details |
| GPC3-T2 CAR-T cell therapy (The Second Affiliated Hospital of Guangzhou Medical University) | Phase 1 Clinical | Second Affiliated Hospital Of Guangzhou Medical University | Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details | |
| Codrituzumab | GC-33; RG-7686; RO-5137382 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd, F. Hoffmann-La Roche Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| GPC-3298306 | GPC-3298306 | Phase 2 Clinical | National Cancer Center Of Japan | Ovarian Neoplasms; Carcinoma, Hepatocellular | Details |
| TAK-102 | TAK-102 | Phase 1 Clinical | Noile-Immune Biotech Inc | Solid tumours; Neoplasms | Details |
| CT-0180 | CT-0180 | Phase 1 Clinical | CARsgen Therapeutics Holdings Ltd | Carcinoma, Hepatocellular | Details |
| GPC3-CAR-T cell therapy | CSG-GPC-3; CAR-GPC3 T-cell; GPC3-CAR/CSG-GPC3; anti-GPC-3 CAR T; KJgpc3-001; CT-011 | Phase 1 Clinical | Solid tumours; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details | |
| B010-A | B010-A | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Carcinoma, Hepatocellular | Details |
| CT-017 | CT-017 | Phase 1 Clinical | Carsgen Biomedicine (Shanghai) Co Ltd | Carcinoma, Hepatocellular | Details |
| CT0181 | CT-0181 | Phase 1 Clinical | CARsgen Therapeutics Holdings Ltd | Carcinoma, Hepatocellular | Details |
| GPC3-targeted CAR-T (Peking University) | Phase 1 Clinical | Peking University | Carcinoma, Hepatocellular | Details | |
| CAR (hYP7)-T cells (National Cancer Institute) | Phase 1 Clinical | National Cancer Institute | Liver Neoplasms; Carcinoma, Hepatocellular | Details | |
| Anti-GPC3 chimeric antigen receptor T cell therapy (Hunan Zhaotai Yongren Biotech/Guangdong Zhaotai Invivo Biomedicine) | Phase 1 Clinical | Hunan Zhaotai Yongren Biotech Co Ltd, Guangdong Zhaotai Invivo Biomedicine Co Ltd | Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details | |
| BOXR-1030 | BOXR-1030 | Phase 2 Clinical | Unum Therapeutics Inc | Liver Neoplasms; Carcinoma, Merkel Cell; Carcinoma, Squamous Cell; Lung Neoplasms; Liposarcoma, Myxoid; Carcinoma, Hepatocellular | Details |
This web search service is supported by Google Inc.
