
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!

This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 30.7 kDa. The protein migrates as 40-120 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
>80% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in 50 mM Tris, 100 mM NaCl, pH8.0 with detergent with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
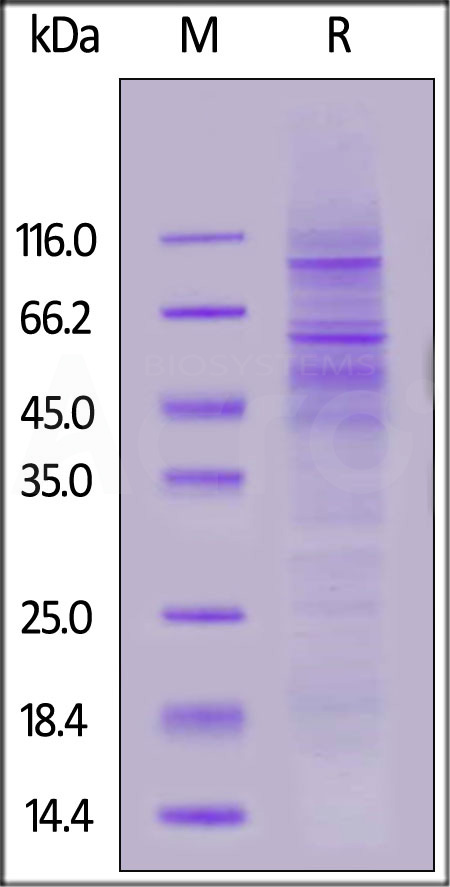
Human PD-1 Full Length, His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 80%.

Immobilized Human PD-1 Full Length, His Tag (Cat. No. PD1-H52H6) at 0.2 μg/mL (100 μL/well) can bind Nivolumab with a linear range of 0.4-3 ng/mL (QC tested).
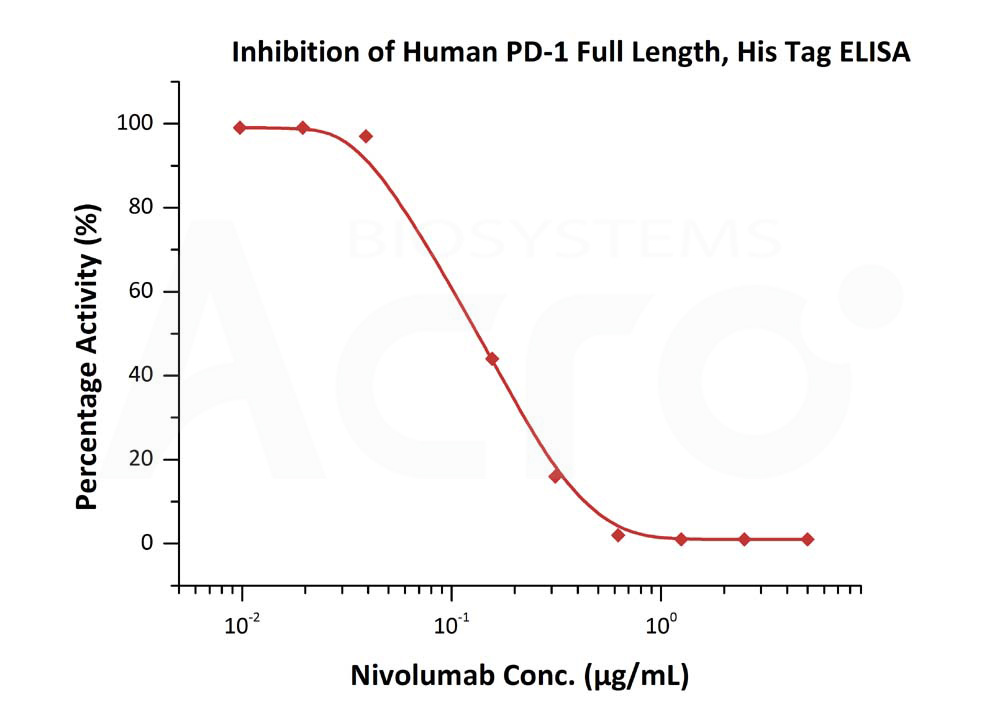
Serial dilutions of nivolumab were added into Human PD-1 Full Length, His Tag (Cat. No. PD1-H52H6) : Human PD-L1, Mouse IgG1 Fc Tag, low endotoxin (Cat. No. PD1-H52A3) binding reactions. The half maximal inhibitory concentration (IC50) is 0.14891 (QC tested).

Immobilized Human PD-1 Full Length, His Tag (Cat. No. PD1-H52H6) at 0.2 μg/mL (100 μL/well) can bind Human PD-L1, Mouse IgG1 Fc Tag, low endotoxin (Cat. No. PD1-H52A3) with a linear range of 0.01-0.313 μg/mL (Routinely tested).

Immobilized Human PD-1 Full Length, His Tag (Cat. No. PD1-H52H6) at 0.2 μg/mL (100 μL/well) can bind Human PD-L2, Mouse IgG1 Fc Tag (Cat. No. PD2-H52A5) with a linear range of 0.5-8 ng/mL (Routinely tested).
Price(USD) : $540.00
Price(USD) : $1900.00
Price(USD) : $4950.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Serplulimab | HLX-10 | Approved | Shanghai Henlius Biotech Inc | 汉斯状, Zerpidio, Han Si Zhuang, HANSIZHUANG | Mainland China | Microsatellite instability-high cancer | Shanghai Henlius Biopharmaceuticals Co Ltd | 2022-03-22 | Triple Negative Breast Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Colorectal Neoplasms; Microsatellite Instability; Microsatellite instability-high cancer; Neoplasms; Solid tumours; Small Cell Lung Carcinoma; Hepatitis B, Chronic; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Liver Neoplasms; Head and Neck Neoplasms | Details |
| Nivolumab/Relatlimab | BMS-986213; BMS-936558/BMS-986016 | Approved | Bristol-Myers Squibb Company | OPDUALAG | United States | Melanoma | Bristol-Myers Squibb Company | 2022-03-18 | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Merkel Cell; Colorectal Neoplasms; Melanoma | Details |
| Zimberelimab | AB-122; GLS-010; WBP-3055 | Approved | Wuxi Biologics (Shanghai) Co Ltd, Harbin Gloria Pharmaceuticals Co Ltd | 誉妥, YUTUO | Mainland China | Hodgkin Disease | Guangzhou Gloria Biosciences Co Ltd | 2021-08-25 | Prostatic Neoplasms, Castration-Resistant; Adenocarcinoma; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Esophageal adenocarcinoma; Endometrial Neoplasms; Carcinoma, Pancreatic Ductal; Sarcoma, Alveolar Soft Part; Lung Neoplasms; Lymphoma, Non-Hodgkin; Carcinoma, Squamous Cell; Colorectal Neoplasms; Breast Neoplasms; Multiple Myeloma; Urinary Bladder Neoplasms; Solid tumours; Neoplasms; Triple Negative Breast Neoplasms; Small Cell Lung Carcinoma; Hodgkin Disease; Glioblastoma; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma, Merkel Cell; Stomach Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms; Ovarian Neoplasms | Details |
| Pucotenlimab | AK-103; HX-008 | Approved | Hanx Biopharmaceutical Co Ltd, Zhongshan Akeso Biopharma Co Ltd | 普佑恒 | Mainland China | Microsatellite instability-high cancer; Mismatch Repair Deficient Cancer | Lepu Biotech Co Ltd | 2022-07-19 | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Mismatch Repair Deficient Cancer; Thyroid Carcinoma, Anaplastic; Triple Negative Breast Neoplasms; Urinary Bladder Neoplasms; Microsatellite instability-high cancer; Colorectal Neoplasms; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Gastrointestinal Neoplasms | Details |
| Cemiplimab | REGN-2810; SAR-439684 | Approved | Libtayo | United States | Carcinoma, Squamous Cell | Regeneron Pharmaceuticals Inc | 2018-09-28 | Lung Neoplasms; Sarcoma; Peritoneal Neoplasms; Diffuse Intrinsic Pontine Glioma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Carcinoma, Brown-Pearce; Mouth Neoplasms; Glioma; Prostatic Neoplasms; Brain metastases; Endometrial Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Mycosis Fungoides; Melanoma; Carcinoma, Hepatocellular; Skin Neoplasms; Solid tumours; HIV Infections; Carcinoma, Bronchogenic; Carcinoma, Basal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Merkel Cell; Hemangiosarcoma; Carcinoma, Renal Cell; Ovarian Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Hodgkin Disease; Papillomavirus Infections; Colonic Neoplasms; Central Nervous System Neoplasms; Multiple Myeloma; Hepatitis B | Details | |
| Cadonilimab | AK-104; AK104 | Approved | Zhongshan Akeso Biopharma Co Ltd | 开坦尼 | Mainland China | Uterine Cervical Neoplasms | Kangfang Pharmaceutical Co Ltd | 2022-06-29 | Nasopharyngeal Carcinoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Uterine Cervical Neoplasms; Esophageal Squamous Cell Carcinoma; DNA Repair-Deficiency Disorders; Urologic Neoplasms; Colorectal Neoplasms; Vulvar Neoplasms; Microsatellite Instability; Solid tumours; Microsatellite instability-high cancer; Triple Negative Breast Neoplasms; Neoplasms; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Vaginal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Lymphoma, T-Cell, Peripheral | Details |
| Prolgolimab | BCD-100 | Approved | Biocad | Forteca | Russian Federation | Melanoma | Biocad | 2020-04-01 | Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Lung Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Tislelizumab | BGB-A317; VDT-482 | Approved | Beigene Ltd | 百泽安, Tevimbra, TEVIMBRA | Mainland China | Hodgkin Disease | Beigene (Shanghai) Biotechnology Co Ltd | 2019-12-26 | Lymphoma, Large-Cell, Anaplastic; Lymphoma, Extranodal NK-T-Cell; Sarcoma; Cholangiocarcinoma; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Colorectal Neoplasms; Esophageal Squamous Cell Carcinoma; Lymphoma; Lymphoma, T-Cell; Nasopharyngeal Carcinoma; Lymphoma, T-Cell, Cutaneous; Lung Neoplasms; Metastatic breast cancer; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Leukemia, Lymphocytic, Chronic, B-Cell; Uterine Cervical Neoplasms; Melanoma; Lymphoma, Large B-Cell, Diffuse; Solid tumours; Liver Neoplasms; Leukemia; Lymphoma, B-Cell, Marginal Zone; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Mismatch Repair Deficient Cancer; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Neoplasms; Pancreatic Neoplasms; Carcinoma, Adenoid Cystic; Digestive System Neoplasms; Urinary Bladder Neoplasms; Microsatellite instability-high cancer | Details |
| Toripalimab | TAB-001; JS-001; JS 001; SO-001; JS-001sc; CHS-007 | Approved | Suzhou Union Biopharm Biosciences Co Ltd, Shanghai Junshi Biosciences Co Ltd | 拓益, LOQTORZI, Tuoyi | Mainland China | Melanoma | Shanghai Junshi Biosciences Co Ltd | 2018-12-17 | Esophageal Squamous Cell Carcinoma; Urinary Bladder Neoplasms; Neuroendocrine Tumors; Rejection of organ transplantation; Nasopharyngeal Carcinoma; Cholangiocarcinoma; Breast Neoplasms; Sarcoma; Colorectal Neoplasms; Urologic Neoplasms; Hodgkin Disease; Lymphoma; Laryngeal Neoplasms; Lung Neoplasms; Epstein-Barr Virus Infections; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Melanoma; Adenocarcinoma; Stomach Neoplasms; Kidney Neoplasms; Solid tumours; Colorectal Neoplasms, Hereditary Nonpolyposis; Biliary Tract Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Pain; Squamous Cell Carcinoma of Head and Neck; Head and Neck Neoplasms; Anus Neoplasms; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Nasopharyngeal Neoplasms; Nasopharyngeal Diseases; Hypopharyngeal Neoplasms; Neoplasms | Details |
| Dostarlimab | WBP-285; TSR-042; ANB-011; GSK-4057190 | Approved | Anaptysbio Inc | JEMPERLI, Jemperli | EU | Endometrial Neoplasms | Glaxosmithkline (Ireland) Ltd | 2021-04-21 | Breast Neoplasms; Melanoma; Neoplasms, Second Primary; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Duodenal Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Endometrial Neoplasms; Carcinoma, Neuroendocrine; Colorectal Neoplasms; Peritoneal Neoplasms; Ampullary Carcinoma; Sarcoma, Clear Cell; Ovarian Neoplasms; Sarcoma; Gestational Trophoblastic Disease; Mesothelioma; Adrenocortical Carcinoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Neoplasms; Rectal Neoplasms; Mismatch Repair Deficient Cancer; Stomach Neoplasms; Head and Neck Neoplasms; Solid tumours | Details |
| Camrelizumab | SHR-1210; INCSHR-1210; HR-301210; SHR01210 | Approved | Jiangsu Hengrui Medicine Co Ltd, Suzhou Suncadia Biopharmaceuticals Co Ltd | 艾立妥, 艾瑞卡, AiRuiKa | Mainland China | Hodgkin Disease | Suzhou Suncadia Biopharmaceuticals Co Ltd | 2019-05-29 | Lymphoma; Breast Neoplasms; Respiratory Tract Neoplasms; Nasopharyngeal Carcinoma; Lymphoma, Extranodal NK-T-Cell; Sarcoma; Cholangiocarcinoma; Colorectal Neoplasms; Genital Neoplasms, Female; Urologic Neoplasms; Carcinoma, Squamous Cell; Endometrial Neoplasms; Urinary Bladder Neoplasms; Paranasal Sinus Neoplasms; Esophageal Squamous Cell Carcinoma; Uterine Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Melanoma; Gastrointestinal Neoplasms; Neoplasm Metastasis; Esophageal Diseases; Small Cell Lung Carcinoma; Carcinoma, Bronchogenic; Ovarian Neoplasms; Solid tumours; Hemorrhagic Fever, Ebola; Biliary Tract Neoplasms; Stomach Neoplasms; Rectal Neoplasms; Thoracic Neoplasms; Bronchial Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Liver Neoplasms; Nasopharyngeal Neoplasms; Lymphoma, Large B-Cell, Diffuse; Hypopharyngeal Neoplasms; Hodgkin Disease; Carcinoma, Transitional Cell; Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neopl | Details |
| Nivolumab | MDX-1106; BMS-936558; BMS-936558-01; ono-0123; ONO-4538 | Approved | Ono Pharmaceutical Co Ltd, Bristol-Myers Squibb Company | 欧狄沃, Opdivo | Japan | Melanoma | Ono Pharmaceutical Co Ltd | 2014-07-04 | Liver Neoplasms; Hematologic Neoplasms; Solid tumours; Drug-Related Side Effects and Adverse Reactions; Head and Neck Neoplasms; Biliary Tract Neoplasms; Tobacco Smoking; Lymphoma, T-Cell, Peripheral; Acquired Immunodeficiency Syndrome; Bone Marrow Neoplasms; Kidney Neoplasms; HIV Infections; Leukemia, Myelogenous, Chronic; Hematopoietic stem cell transplantation (HSCT); Ependymoma; Medulloblastoma; Colorectal Neoplasms, Hereditary Nonpolyposis; Leiomyosarcoma; Ovarian Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Anus Neoplasms; Mismatch Repair Deficient Cancer; Leukemia, Lymphoid; Leukoplakia, Oral; Carcinoma; Carcinoma, Merkel Cell; Giant Cell Arteritis; Polycythemia Vera; Inflammatory Bowel Diseases; Dermatomyositis; Thoracic Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Pancreatic Neoplasms; Sepsis; Hodgkin Disease; Autoimmune Diseases; Myelodysplastic Syndromes; Coronavirus D | Details |
| Sintilimab | IBI-308 | Approved | Innovent Biologics(Suzhou) Co Ltd | Tyvyt, 达伯舒 | Mainland China | Hodgkin Disease | Innovent Biologics(Suzhou) Co Ltd | 2018-12-24 | Lung Diseases; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Lung Neoplasms; Endometrial Neoplasms; Neoplasms, Unknown Primary; Lymphoma, Non-Hodgkin; Esophageal Squamous Cell Carcinoma; Colorectal Neoplasms; Lymphoma, Extranodal NK-T-Cell; Sarcoma; Lymphoma, T-Cell, Peripheral; Respiratory Tract Diseases; Nasopharyngeal Neoplasms; Neoplasms; Hodgkin Disease; Thoracic Neoplasms; Hemangiosarcoma; Esophageal Neoplasms; Stomach Neoplasms; Solid tumours; Liver Neoplasms | Details |
| Retifanlimab | MGA-012; INCMGA-0012; INCMGA-00012 | Approved | Macrogenics Inc | ZYNYZ | United States | Carcinoma, Merkel Cell | Incyte Corp | 2023-03-22 | Carcinoma, Squamous Cell; Neuroendocrine Tumors; Liposarcoma; Breast Neoplasms; Lymphoma, Follicular; Sarcoma; Astrocytoma; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Glioma; Digestive System Neoplasms; Endometrial Neoplasms; Esophageal adenocarcinoma; Penile Neoplasms; Neoplasm Metastasis; Carcinoma, Endometrioid; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Melanoma; Neoplasms; Solid tumours; Ovarian Neoplasms; Lymphoma, B-Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Anus Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Merkel Cell; Head and Neck Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Malignant Carcinoid Syndrome; Lymphoma, Large B-Cell, Diffuse; Glioblastoma; Prostatic Neoplasms, Castration-Resistant; Oligodendroglioma; Urinary Bladder Neoplasms | Details |
| Penpulimab | AK-105 | Approved | Zhongshan Akeso Biopharma Co Ltd | 安尼可, ANNIKO | Mainland China | Hodgkin Disease | Zhengda Tianqing Kangfang (Shanghai) Biomedical Technology Co Ltd | 2021-08-03 | Neuroendocrine Tumors; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Lymphoma, Non-Hodgkin; Carcinoma, Squamous Cell; Urologic Neoplasms; Nasopharyngeal Carcinoma; Solid tumours; Microsatellite instability-high cancer; Neoplasms; Hodgkin Disease; Small Cell Lung Carcinoma; Esophageal Neoplasms; Thoracic Neoplasms; Liver Neoplasms; Head and Neck Neoplasms | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Budigalimab | ABBV-181 | Phase 3 Clinical | Abbvie Inc | HIV Infections; Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Cetrelimab | JNJ-63723283; JNJ-3283 | Phase 3 Clinical | Johnson & Johnson | Solid tumours; Hepatitis B, Chronic; Neoplasms; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Neuroendocrine; Carcinoma, Small Cell; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Nofazinlimab | CS-1003 | Phase 3 Clinical | Cstone Pharmaceuticals | Solid tumours; Neoplasms; Lymphoma; Carcinoma, Hepatocellular | Details |
| Sasanlimab | RN-888; PF-06801591; PF-6801591 | Phase 3 Clinical | Pfizer Inc | Endometrial Neoplasms; Lymphoma, Large B-Cell, Diffuse; Digestive System Neoplasms; Urinary Bladder Neoplasms; Urologic Diseases; Sarcoma; Prostatic Neoplasms; Bile Duct Neoplasms; Urologic Neoplasms; Colorectal Neoplasms; Triple Negative Breast Neoplasms; Urogenital Neoplasms; Carcinoma, Squamous Cell; Gastrointestinal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Fructose Intolerance; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Kidney Neoplasms; Liver Neoplasms; Head and Neck Neoplasms; Lymphoma, T-Cell, Peripheral; Skin Melanoma; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma; Solid tumours; Squamous Cell Carcinoma of Head and Neck; Pancreatic Neoplasms; Neoplasms; Neoplasms, Glandular and Epithelial; Kidney Diseases; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Hodgkin Disease | Details |
| BAT-1308 | BAT-1308 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Solid tumours; Triple Negative Breast Neoplasms; Endometrial Neoplasms; Uterine Cervical Neoplasms | Details |
| Genolimzumab | CBT-501; APL-501; APL501; GB226; GB-226 | Phase 3 Clinical | Cbt, Genor Biopharma Co Ltd, Apollomics Inc | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular; Lymphoma, Non-Hodgkin; Sarcoma, Alveolar Soft Part; Thymoma; Lymphoma; Neoplasms, Unknown Primary; Lymphoma, T-Cell, Peripheral; Thymus Neoplasms; Microsatellite Instability; Hodgkin Disease; Carcinoma, Renal Cell; Mismatch Repair Deficient Cancer; Lymphoma, B-Cell; Solid tumours | Details |
| Volrustomig | MEDI-5752; MEDI5752 | Phase 3 Clinical | Medimmune Llc | Biliary Tract Neoplasms; Solid tumours; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoma, Renal Cell; Mesothelioma; Sarcoma; Bile Duct Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| BCD-217 | BCD-217 | Phase 3 Clinical | Biocad | Melanoma | Details |
| Tebotelimab | PD-1 X LAG-3; MGD-013 | Phase 3 Clinical | Macrogenics Inc | Triple Negative Breast Neoplasms; Adenocarcinoma; Carcinoma, Hepatocellular; Melanoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Neoplasm Metastasis; Endometrial Neoplasms; Cholangiocarcinoma; Neoplasms; Solid tumours; Small Cell Lung Carcinoma; Esophageal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Liver Neoplasms; Biliary Tract Neoplasms; Hematologic Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms | Details |
| Rulonilimab | F-520 | Phase 3 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, T-Cell, Peripheral; Solid tumours; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Central Nervous System Lymphoma; Uterine Cervical Neoplasms | Details |
| Pembrolizumab biosimilar (Amgen) | ABP 234; ABP-234 | Phase 3 Clinical | Amgen Inc | Carcinoma, Non-Small-Cell Lung | Details |
| Pembrolizumab biosimilar (Laboratorio Elea Phoenix Sa) | MB-12; MB12 | Phase 3 Clinical | Laboratorio Elea Phoenix Sa | Carcinoma, Non-Small-Cell Lung | Details |
| Pembrolizumab biosimilar(R-Pharm) | RPH-075 | Phase 3 Clinical | R-Pharm | Skin Melanoma; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
| Pembrolizumab Biosimilar (Samsung Bioepis ) | SB27; SB-27 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| GME-751 | GME751; GME-751 | Phase 3 Clinical | Sandoz | Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| BAT-3306 | BAT3306; BAT-3306 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| PD-1 Inhibitor Therapy(Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University) | Phase 3 Clinical | Sun Yat-Sen Memorial Hospital Of Sun Yat-Sen University | Rejection of liver transplantation; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Hepatocellular | Details | |
| Nivolumab biosimilar(Amgen) | ABP-206 | Phase 3 Clinical | Amgen Inc | Melanoma | Details |
| Pembrolizumab biosimilar (biocad) | BCD-201 | Phase 3 Clinical | Biocad | Solid tumours; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| MK-3475A | MK-3475A | Phase 3 Clinical | Merck Sharp & Dohme Corp | Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Lung Neoplasms; Carcinoma, Squamous Cell; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Pembrolizumab/Vibostolimab | MK-7684A | Phase 3 Clinical | Merck & Co Inc | Urinary Bladder Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Gallbladder Neoplasms; Endometrial Neoplasms; Lung Neoplasms; Cholangiocarcinoma; Ovarian Neoplasms; Triple Negative Breast Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Hematologic Neoplasms | Details |
| Rilvegostomig | AZD-2936 | Phase 3 Clinical | Astrazeneca Plc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Bile Duct Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Pembrolizumab/Quavonlimab | MK-1308A | Phase 3 Clinical | Merck Sharp & Dohme Corp, Msd Ireland (Carlow), MSD R&D(China)Co Ltd | Solid tumours; Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Colorectal Neoplasms; Carcinoma, Hepatocellular; Melanoma | Details |
| Mavezelimab/Pembrolizumab | MK-4280A; MK-4820A | Phase 3 Clinical | Msd Ireland (Carlow) Merck Sharp & Dohme Corp, MSD R&D(China)Co Ltd | Solid tumours; Carcinoma, Renal Cell; Hodgkin Disease; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Colorectal Neoplasms; Carcinoma, Squamous Cell; Endometrial Neoplasms | Details |
| Nivolumab biosimilar (Shandong Boan Biotechnology) | LY-01015; BA-1104 | Phase 3 Clinical | Liver Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Urinary Bladder Neoplasms; Mesothelioma; Glioma; Esophageal Squamous Cell Carcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung | Details | |
| Spartalizumab | PDR-001 | Phase 3 Clinical | Novartis Pharma Ag | Esophageal Squamous Cell Carcinoma; Chordoma; Digestive System Neoplasms; Neuroendocrine Tumors; Nasopharyngeal Carcinoma; Breast Neoplasms; Sarcoma; Prostatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Primary Myelofibrosis; Bone Marrow Diseases; Esophageal adenocarcinoma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Microsatellite instability-high cancer; Carcinoma, Pancreatic Ductal; Lung Neoplasms; Lymphoma; Sarcoma, Alveolar Soft Part; Leukemia, Myeloid, Acute; Thyroid Neoplasms; Metastatic breast cancer; Uterine Neoplasms; Adenocarcinoma; Persistent Fetal Circulation Syndrome; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Uterine Cervical Neoplasms; Lymphoma, Large B-Cell, Diffuse; Solid tumours; Ovarian Neoplasms; Liver Neoplasms; Leukemia, Myeloid; Leukemia; Kidney Neoplasms; Preleukemia; Hematologic Diseases; Carcinoma, Renal Cell; Carcinoma; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Anus Neoplasms; Triple Negati | Details |
| CD19-PD1-CAR T cell therapy (Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Lymphoma, B-Cell | Details | |
| dPD-1 hCD19 CART cell therapy (Innovative Cellular Therapeutics) | ICTCAR-014 | Phase 2 Clinical | Innovative Cellular Therapeutics Co Ltd | Lymphoma, Non-Hodgkin | Details |
| Pimivalimab | JTX-4014 | Phase 2 Clinical | Jounce Therapeutics Inc | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| MAX-10181 | MAX-10181; MAX-1 | Phase 2 Clinical | Maxinovel Pharmaceuticals Co Ltd | Solid tumours; Neoplasms | Details |
| Tobemstomig | RO-7247669 | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Liver Neoplasms; Carcinoma; Carcinoma, Renal Cell; Neoplasms; Carcinoma, Transitional Cell; Breast Neoplasms; Esophageal Squamous Cell Carcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| SI-B003 | SI-B003 | Phase 2 Clinical | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Breast Neoplasms; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Neoplasm Metastasis; Melanoma | Details | |
| Multiple target antigen stimulating cell therapy (HengRui YuanZheng Biotechnology) | Phase 2 Clinical | Hengrui Yuanzheng (Shanghai) Biotechnology Co Ltd | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Neoplasms; Liver Diseases | Details | |
| Vudalimab | XmAb-20717; XmAb-717; XmAb 717 | Phase 2 Clinical | Xencor Inc | Thymoma; Adenocarcinoma, Clear Cell; Cholangiocarcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Vulvar Neoplasms; Prostatic Neoplasms; Astrocytoma; Colorectal Neoplasms; Endometrial Neoplasms; Microsatellite instability-high cancer; Carcinoma, Neuroendocrine; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Thyroid Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Ovarian Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Carcinoma, Basal Cell; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Biliary Tract Neoplasms; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Hodgkin Disease; Adenoma, Acidophil; Colonic Neoplasms; Mesothelioma; Adnexal Diseases; Prostatic Neoplasms, Castration-Resistant | Details |
| Recombinant humanized anti-PD-1 monoclonal antibody (Shanghai CP Guojian) | 609-A; SSGJ-609A; SSGJ-609-A | Phase 2 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Solid tumours; Sarcoma; Breast Neoplasms | Details |
| Lorigerlimab | MGD-019; AEX1344 | Phase 2 Clinical | Macrogenics Inc | Solid tumours; Liver Neoplasms; Skin Melanoma; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Pancreatic Ductal; Uterine Cervical Neoplasms; Neoplasm Metastasis; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| PD-1 knockout EBV-CTL (Nanjing Medical University) | Phase 2 Clinical | Nanjing Medical University | Stomach Neoplasms; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Nasopharyngeal Neoplasms; Lymphoma | Details | |
| Izuralimab | XmAb-23104; XmAb-104 | Phase 2 Clinical | Xencor Inc | Sarcoma; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Endometrial Neoplasms; Colorectal Neoplasms; Breast Neoplasms; Nasopharyngeal Carcinoma; Solid tumours; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms | Details |
| Peresolimab | LY-3462817 | Phase 2 Clinical | Eli Lilly And Company | Arthritis, Rheumatoid; Autoimmune Diseases; Connective Tissue Diseases; Immune System Diseases; Joint Diseases; Psoriasis; Musculoskeletal Diseases; Rheumatic Diseases; Arthritis | Details |
| Lomvastomig | RG-7769; RO-7121661 | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms; Small Cell Lung Carcinoma; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| MiHA-loaded PD-L silenced DC vaccination (Radboud University) | Phase 2 Clinical | Radboud University Nijmegen | Hematologic Neoplasms; Colorectal Neoplasms | Details | |
| ZG-005 | ZG-005 | Phase 2 Clinical | Gensun Biopharma Inc | Solid tumours; Neoplasms; Carcinoma, Neuroendocrine; Uterine Cervical Neoplasms | Details |
| T-3011 | T-3 (ImmVira Pharma); MVR-T3011; T3011; MVR-T3011 IT; MVR-T3011 IV; B015; B-015 | Phase 2 Clinical | Immvira Co Ltd | Breast Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Squamous Cell; Lymphoma; Endometrial Neoplasms; Lung Neoplasms; Colorectal Neoplasms; Sarcoma; Liver Neoplasms; Ascites; Mesothelioma; Small Cell Lung Carcinoma; Skin Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Head and Neck Neoplasms; Solid tumours; Ovarian Neoplasms | Details |
| Anti-CTLA-4 and PD-1 CAR-T cell therapy (Shanghai Cell Therapy Research Institute) | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Neoplasms | Details | |
| TY-101 | TY-101; TY101 | Phase 2 Clinical | Tayu Huaxia Biotech Medical Group Co Ltd | Solid tumours; Lymphoma | Details |
| BCMA-PD1 CAR T cell therapy (General Hospital of the People's Liberation Army) | Phase 2 Clinical | People'S Liberation Army General Hospital Military Service | Multiple Myeloma | Details | |
| Sabestomig | AZD-7789 | Phase 2 Clinical | AstraZeneca Ag | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Hodgkin Disease; Digestive System Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Gastrointestinal Neoplasms | Details |
| TC-510 | TC-510 | Phase 2 Clinical | Tcr2 Therapeutics Inc | Ovarian Neoplasms; Solid tumours; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Mesothelioma; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| Anti-PD-1 Antibody(Jiangxi Provincial Cancer Hospital) | Phase 2 Clinical | Jiangxi Provincial Cancer Hospital | Nasopharyngeal Carcinoma | Details | |
| Ezabenlimab | BI-754091 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Sarcoma; Melanoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Lung Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Carcinoma, Neuroendocrine; Carcinoma, Pancreatic Ductal; Colorectal Neoplasms; Head and Neck Neoplasms; Colonic Neoplasms; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Neoplasms; Pancreatic Neoplasms; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Liver Neoplasms; Biliary Tract Neoplasms; Solid tumours | Details |
| PD-1 Inhibitor Therapy(Affiliated Hospital of Nantong University) | Phase 2 Clinical | Affiliated Hospital Of Nantong University | Esophageal Squamous Cell Carcinoma | Details | |
| PD-1 inhibitor therapy(Shanghai Chest Hospital) | Phase 2 Clinical | Shanghai Chest Hospital | Esophageal Neoplasms; Esophageal Squamous Cell Carcinoma | Details | |
| Recombinant humanized anti-PD-1 monoclonal antibody (Anhui Anke Biotechnology) | Phase 2 Clinical | Anhui Anke Biotechnology (Group) Co Ltd | Esophageal Neoplasms; Carcinoma, Transitional Cell; Triple Negative Breast Neoplasms; Neoplasms; Lymphoma; Lung Neoplasms | Details | |
| PD-1 Monoclonal Antibody(Sixth Affiliated Hospital Sun Yat-Sen University) | Phase 2 Clinical | Sixth Affiliated Hospital Sun Yat-Sen University | Neoplasms; Colorectal Neoplasms | Details | |
| PD-1 Inhibitor Therapy(Beijing YouAn Hospital) | Phase 2 Clinical | Beijing Youan Hospbeijing Youan Hospital,Capital Medical Universityital,Capital Medical University, Beijing Gene Key Life Technology Co Ltd | Carcinoma, Hepatocellular | Details | |
| Autologous PD-1 antibody expressing mesothelin-targeted CAR-Tcells therapy (Shanghai Cell Therapy Research Institute) | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Solid tumours | Details | |
| SAR-445877 | SAR445877; SAR-445877; KD050; KD-050 | Phase 2 Clinical | Sanofi, Kadmon Corporation Llc | Solid tumours | Details |
| Peramprizumab | Phase 2 Clinical | Sun Yat-Sen University | Nasopharyngeal Carcinoma | Details | |
| IBI-363 | IBI-363 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms; Lymphoma; Melanoma | Details |
| EBV-specific TCR-T cell with anti-PD1 auto-secreted element | Phase 2 Clinical | Tcrcure Biopharma Ltd | Squamous Cell Carcinoma of Head and Neck | Details | |
| Anti-CTLA-4/PD-1 expressing EGFR-CAR-T | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Solid tumours | Details | |
| HerinCAR-PD1 | Phase 2 Clinical | Ningbo Cancer Hospital | Solid tumours | Details | |
| IAP-0971 | IAP-0971 | Phase 2 Clinical | SunHo (China) BioPharmaceutical Co Ltd | Solid tumours; Neoplasms; Urinary Bladder Neoplasms | Details |
| Rosnilimab | ANB-030 | Phase 2 Clinical | Anaptysbio Inc | Alopecia Areata; Arthritis, Rheumatoid; Colitis, Ulcerative | Details |
| TQB-2868 | TQB-2868 | Phase 2 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Neoplasms; Pancreatic Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details |
| Pidilizumab | MDV-9300; CT-011 | Phase 2 Clinical | Curetech Ltd | Ovarian Neoplasms; Carcinoma, Renal Cell; Lymphoma, Large B-Cell, Diffuse; Pancreatic Neoplasms; Colonic Neoplasms; Multiple Myeloma; Prostatic Neoplasms; Breast Neoplasms; Sarcoma; Colorectal Neoplasms; Lymphoma; Lymphoma, Non-Hodgkin; Hepatitis C, Chronic; Melanoma; Carcinoma, Hepatocellular | Details |
| Pradusinstobart | LVGN-3616; SSI-361 | Phase 2 Clinical | Lyvgen Biopharma(HK)Ltd | Head and Neck Neoplasms; Solid tumours; Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Papillomavirus Infections; Uveal melanoma; Sarcoma; Carcinoma, Hepatocellular; Neoplasm Metastasis | Details |
| LBL-015 | LBL-015 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Solid tumours | Details |
| EMB-02 | EMB-02 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Solid tumours | Details |
| Acrixolimab | YBL-006; YBL 006 | Phase 2 Clinical | Y-biologics Inc | Solid tumours | Details |
| PD-1 knockout engineered T cells (Anhui Kedgene Biotechnology) | Phase 2 Clinical | Anhui Korton Biological Technology Co Ltd, Hangzhou Cancer Hospital | Esophageal Neoplasms; Carcinoma, Hepatocellular | Details | |
| Lipustobart | LZM-009 | Phase 2 Clinical | Livzon(Group) Pharmaceutical Factory | Solid tumours; Thymoma; Carcinoma, Non-Small-Cell Lung | Details |
| PD-1 knockout engineered T cells (Chengdu MedGenCell) | Phase 2 Clinical | West China Hospital Of Sichuan University, Chengdu Medgencell | Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma | Details | |
| HX-009 | HX-009 | Phase 2 Clinical | Hanx Biopharmaceutical Co Ltd, Wuhan Hanxiong Biotechnology Co Ltd | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Lymphoma | Details |
| MEDI-0680 | AMP-514; MEDI-0680 | Phase 2 Clinical | Medimmune | Lymphoma, B-Cell; Kidney Neoplasms; Carcinoma, Renal Cell; Neoplasms | Details |
| PE0105 | PE-0105 | Phase 1 Clinical | Shanghai Yunyi Health Technology Development Co Ltd | Solid tumours; Neoplasms | Details |
| Recombinant human PD-1 antibody herpes simplex virus | Phase 1 Clinical | Yangsheng Tang Co Ltd, Xiamen University | Liver Neoplasms; Solid tumours; Head and Neck Neoplasms; Neoplasms; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Colorectal Neoplasms; Brain metastases; Lung Neoplasms; Melanoma; Uterine Cervical Neoplasms | Details | |
| GX-P1 | GX-P1 | Phase 1 Clinical | Genexine Inc | Autoimmune Diseases | Details |
| Fidasimtamab | IBI-315 | Phase 1 Clinical | Hanmi Pharmaceutical Co Ltd, Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| Zeluvalimab | AMG-404 | Phase 1 Clinical | Amgen Inc | Solid tumours; Small Cell Lung Carcinoma; Prostatic Neoplasms, Castration-Resistant; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| CC-90006 | C-90006; CC-90006 | Phase 1 Clinical | Anaptysbio Inc | Autoimmune Diseases; Psoriasis | Details |
| RO-7284755 | RO-7284755 | Phase 1 Clinical | F. Hoffmann-La Roche Ag | Solid tumours; Neoplasms | Details |
| EGFRvIII-directed CAR-T cell tharapy (Novartis/University of Pennsylvania) | LXF-821 | Phase 1 Clinical | University Of Pennsylvania, Novartis Pharma Ag | Glioblastoma | Details |
| Sym-021 | Sym-021; S-95016; Sym021 | Phase 1 Clinical | Symphogen A/S | Solid tumours; Lymphoma; Neoplasm Metastasis | Details |
| ONO-4685 | ONO-4685 | Phase 1 Clinical | Merus Nv | Psoriasis; Lymphoma, T-Cell; Plaque psoriasis | Details |
| RB-0004 | RB-0004 | Phase 1 Clinical | Reyoung Pharmaceutical Co Ltd | Solid tumours; Lymphoma | Details |
| CMAB-819 | CMAB8-19 | Phase 1 Clinical | Sinomab Bioscience Ltd | Squamous Cell Carcinoma of Head and Neck; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Taminadenant | NIR-178; PBF-509 | Phase 1 Clinical | Palobiofarma Sl | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Carcinoma, Renal Cell; Carcinoma; Triple Negative Breast Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Colorectal Neoplasms; Parkinson Disease; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| JS-207 | JS-207 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Neoplasms | Details |
| Mableukin 2PD1 (Anwita Biosciences) | AWT020; AWT-020 | Phase 1 Clinical | Anwita Biosciences Inc | Neoplasms; Autoimmune Diseases of the Nervous System | Details |
| ASKG-915 | ASKG-915 | Phase 1 Clinical | Askgene Pharma | Solid tumours; Neoplasms | Details |
| CTX-8371 | CTX-8371 | Phase 1 Clinical | Compass Therapeutics LLC | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Hodgkin Disease; Triple Negative Breast Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Reozalimab | IBI-318; LY-3434172; LY3434172 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd, Eli Lilly And Company | Neoplasms; Small Cell Lung Carcinoma; Lymphoma, Extranodal NK-T-Cell; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details |
| BZT-2312 | BZT-2312; Fast CAR T cells | Phase 1 Clinical | Shanghai Cell Therapy Group Co Ltd | Solid tumours | Details |
| BZE-2209 | BZE-2209; BZE2209; αPD1/CTLA4-MSLN-CAR T Cells | Phase 1 Clinical | Shanghai Cell Therapy Group Co Ltd | Solid tumours | Details |
| BCD-263 | BCD-263 | Phase 1 Clinical | Biocad | Melanoma | Details |
| QL-2107 | QL2107; QL-2107 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| STW204/Gotistobart | AI-061 | Phase 1 Clinical | Oncoc4 Inc | Colorectal Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Fallopian Tube Neoplasms; Bile Duct Neoplasms; Carcinoma, Renal Cell; Peritoneal Neoplasms; Digestive System Neoplasms; Urinary Bladder Neoplasms; Stomach Neoplasms; Anus Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Cystadenocarcinoma, Serous | Details |
| INCA33890 | INCA33890; INCA-33890 | Phase 1 Clinical | Incyte Corp | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| INCA-32459 | INCA-32459 | Phase 1 Clinical | Incyte Corp, Merus Nv | Neoplasms | Details |
| JNJ-67484703 | JNJ-67484703; JNJ-4703 | Phase 1 Clinical | Janssen Research & Development Llc | Arthritis, Rheumatoid | Details |
| KY-0118 | KY-0118; KY0118 | Phase 1 Clinical | Novatim Immune Therapeutics (Zhejiang) Co Ltd | Solid tumours; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Neoplasms; Pancreatic Neoplasms; Colorectal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Sym-024 | Sym-024; S-95024 | Phase 1 Clinical | Symphogen A/S | Solid tumours; Neoplasm Metastasis | Details |
| SOT-201 | SOT-201 | Phase 1 Clinical | Sotio | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| Pegylated recombinant human granulocyte colony stimulating factor(Sun Yat-sen University) | Phase 1 Clinical | Sun Yat-Sen University | Hematologic Neoplasms; Nasopharyngeal Carcinoma | Details | |
| ABBV-1882 | ABBV-1882 | Phase 1 Clinical | Abbvie Inc | HIV Infections | Details |
| CDX-585 | CDX-585 | Phase 1 Clinical | Boaoxin Biotechnology(Nanjing) Co Ltd, Celldex Therapeutics | Liver Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms; Solid tumours; Carcinoma, Renal Cell; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AK-129 | AK-129 | Phase 1 Clinical | Zhongshan Akeso Biopharma Co Ltd | Neoplasms; Hodgkin Disease | Details |
| AK-131 | AK-131 | Phase 1 Clinical | Zhongshan Akeso Biopharma Co Ltd | Solid tumours; Neoplasms | Details |
| 89Zr-N-sucDf-pembrolizumab | Phase 1 Clinical | Merck & Co Inc, Umcg The University Medical Center Groningen | Melanoma; Carcinoma, Non-Small-Cell Lung | Details | |
| 706-3SBio | SSGJ-706; 706 anti -PD1/PD -L1 BsAb; 706 anti-PD1/PD-L1 Bispecific antibody | Phase 1 Clinical | 3sbio Inc | Solid tumours; Composite Lymphoma; Lymphoma | Details |
| Latikafusp | AMG-256 | Phase 1 Clinical | Amgen Inc | Solid tumours | Details |
| Recombinant human anti-PD-1 monoclonal antibody(Hec Biological) | Phase 1 Clinical | Dongguan City Hec Biological Medicine Res And Development Co Ltd | Stomach Neoplasms; Esophageal adenocarcinoma | Details | |
| RG-6279 | RG-6279 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours | Details |
| CA-170 | CA-170; AUPM-170 | Phase 1 Clinical | Aurigene | Solid tumours | Details |
| SHR-1901 | SHR-1901 | Phase 1 Clinical | Suzhou Suncadia Biopharmaceuticals Co Ltd | Neoplasms | Details |
| SSGJ-705 | 705; SSGJ-705 | Phase 1 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Solid tumours; Neoplasms | Details |
| Anti-PD1/anti-TIM3 bispecific antibody (L&L vision biopharmaceuticals) | Bis-5 | Phase 1 Clinical | L&L Biopharma Co Ltd | Solid tumours; Neoplasms | Details |
| CD19 PD-1/CD28 CAR-T Cell Therapy (Second Affiliated Hospital School Of Zhejiang University School Of Medicine) | Phase 1 Clinical | Second Affiliated Hospital Of Zhejiang University School Of Medicine | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma | Details | |
| IBI-321 | IBI-321 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| PH-762 | PH-762; PH762-ACT; RXI 762-ACT | Phase 1 Clinical | Phio Pharmaceuticals Corp | Skin Melanoma; Squamous Cell Carcinoma of Head and Neck; Skin Neoplasms; Small Cell Lung Carcinoma; Breast Neoplasms; Genital Neoplasms, Female; Colorectal Neoplasms; Carcinoma, Squamous Cell; Urogenital Neoplasms; Lung Neoplasms; Melanoma | Details |
| JS-201 | JS-201 | Phase 1 Clinical | Shanghai Junshi Biosciences Co Ltd | Neoplasms | Details |
| IMU-201 | IMU-201 | Phase 1 Clinical | Imugene Ltd | Adenocarcinoma of Lung; Carcinoma, Large Cell; Carcinoma, Squamous Cell; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CD-200-AR-L | CD-200-AR-L; hP-1-A-8 | Phase 1 Clinical | University Of Minnesota, OX2 Therapeutics | Glioblastoma; Glioma | Details |
| PF-07209960 | PF-07209960 | Phase 1 Clinical | Pfizer Inc | Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| GNR-051 | GNR-051 | Phase 1 Clinical | Generium Pharmaceuticals | Carcinoma, Renal Cell; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| IBI-319 | IBI-319 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Neoplasms | Details |
| NWY-001(Biocytogen Pharmaceuticals) | NWY001(Biocytogen Pharmaceuticals); YH008; YH-008; NWY-001(Biocytogen Pharmaceuticals) | Phase 1 Clinical | Eucure Pharmaceutical Technology (Beijing) Co Ltd, Biocytogen Pharmaceuticals (Beijing) Co Ltd | Solid tumours; Hematologic Neoplasms; Neoplasms | Details |
| VT1093 | VT1093; VT-1093 | Phase 1 Clinical | Beijing Weiyuan Likang Biotechnology Co Ltd | Solid tumours | Details |
| αPD1-MSLN-CAR T cell therapy (Shanghai Cell Therapy Group Co.,Ltd) | BZDS1901; BZDS-1901; BZD1901 | Phase 1 Clinical | Shanghai Cell Therapy Group Co Ltd | Ovarian Neoplasms; Solid tumours; Mesothelioma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant humanized anti-PD-1 monoclonal antibody (Shanxi Wichida Bright Pharmaceutical) | Phase 1 Clinical | Shanxi Weiqidaguangming Pharmaceutical Co Ltd | Solid tumours | Details | |
| MW-11 | MW-11; 9MW1111; 9-MW1111; 9MW-1111; 9-MW-1111 | Phase 1 Clinical | Mabwell (Shanghai) Bioscience Co Ltd | Solid tumours; Breast Neoplasms | Details |
| STW204 | STW-204; AI025; AI-025 | Phase 1 Clinical | Suzhou Stainwei Biotech Inc | Solid tumours; Neoplasms | Details |
| Rituximab/Paclitaxel | AR-160 | Phase 1 Clinical | Mayo Clinic | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| 64Cu-DOTA-nivolumab (Bristol-Myers Squibb/Ono Pharmaceutical) | Clinical | Ono Pharmaceutical Co Ltd, Bristol-Myers Squibb Company | Melanoma; Carcinoma, Non-Small-Cell Lung | Details | |
| INSIX RA (Indus Biotech) | Clinical | Indus Biotech Pvt Ltd | Arthritis, Rheumatoid; Inflammation | Details | |
| Treprilimab | Clinical | Sun Yat-Sen University | Oropharyngeal Neoplasms | Details | |
| PD-1 Inhibitor Therapy(Hunan Cancer Hospital) | Hunan Cancer Hospital | Details |
This web search service is supported by Google Inc.







