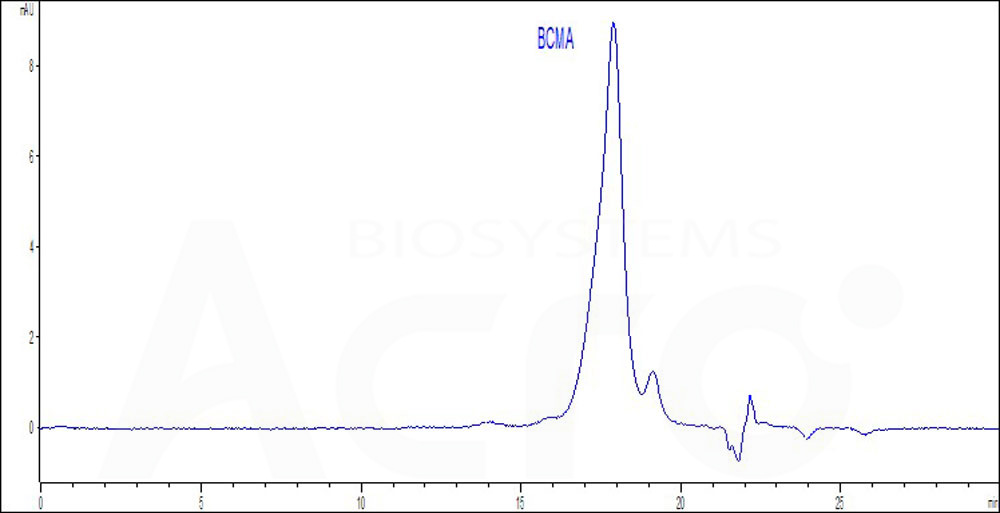
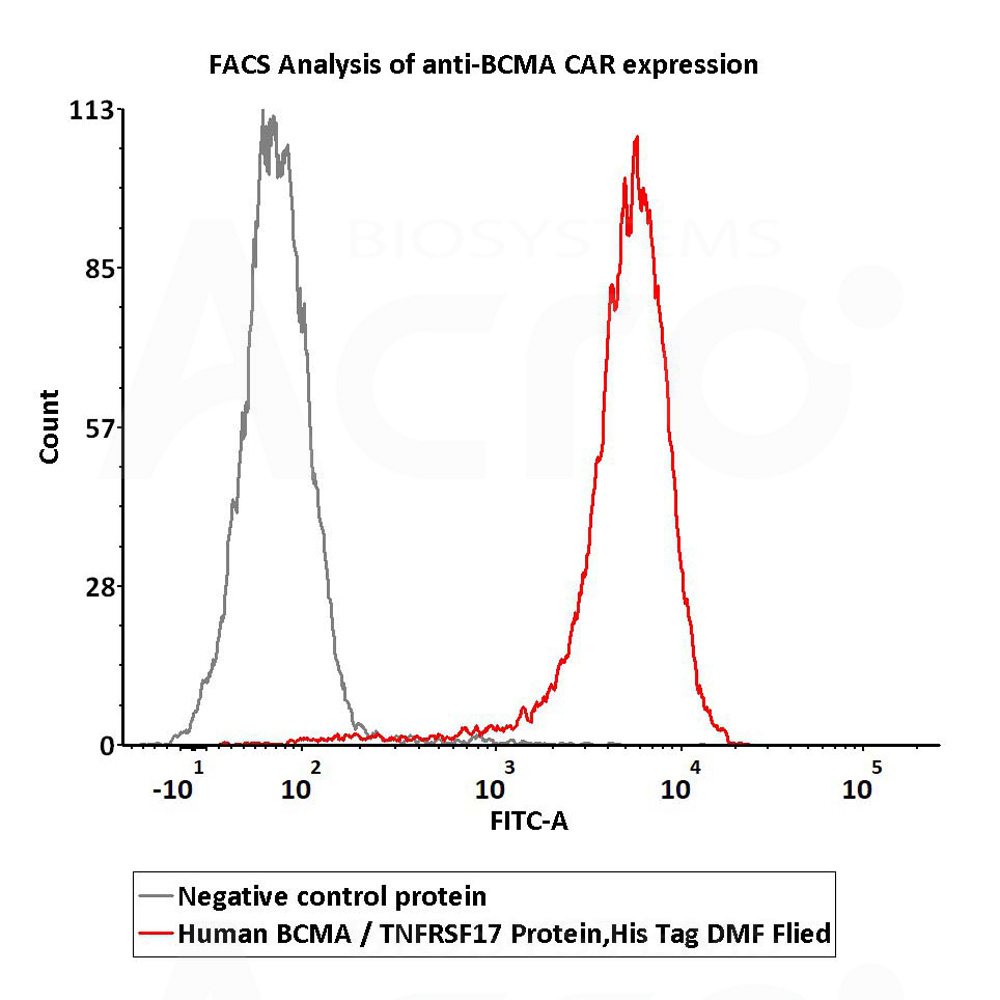
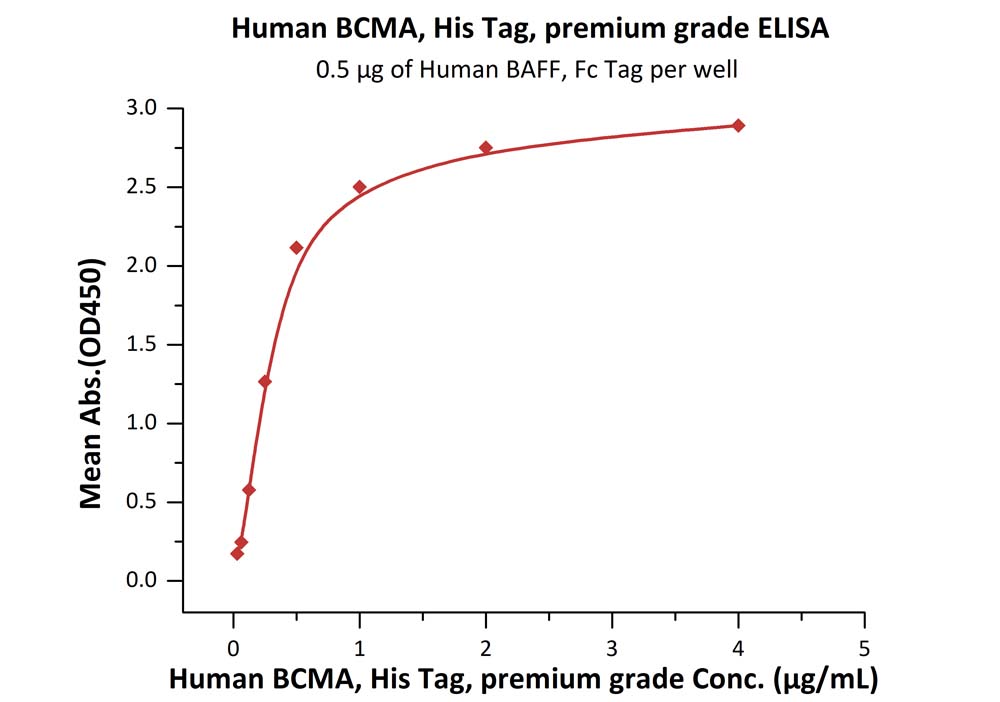
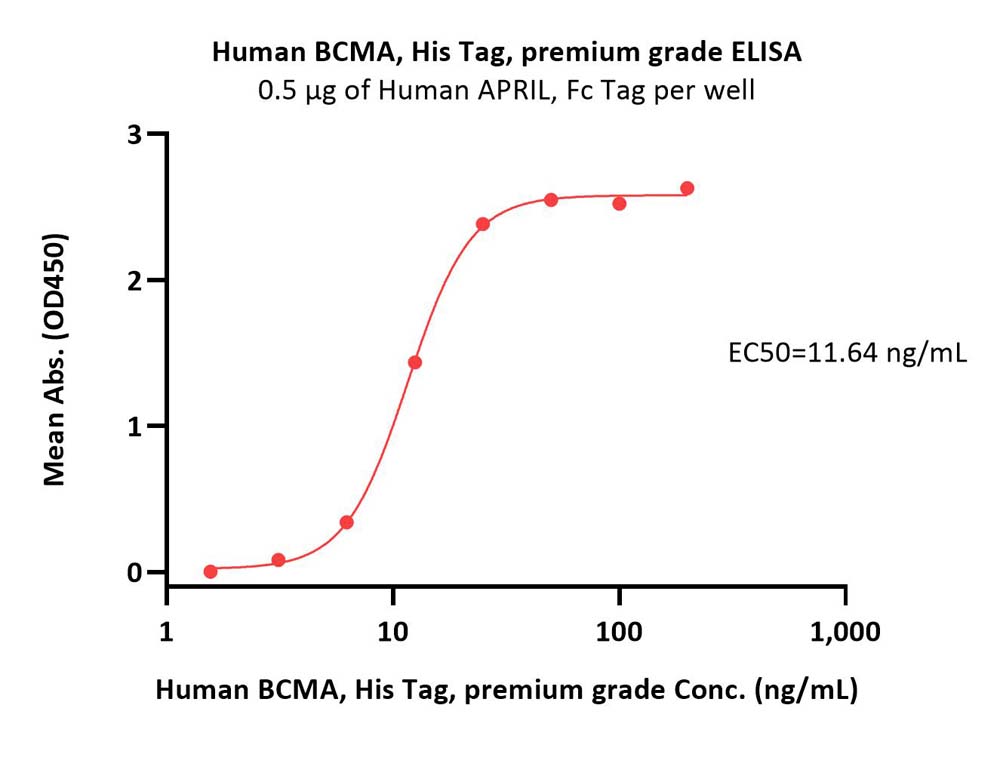
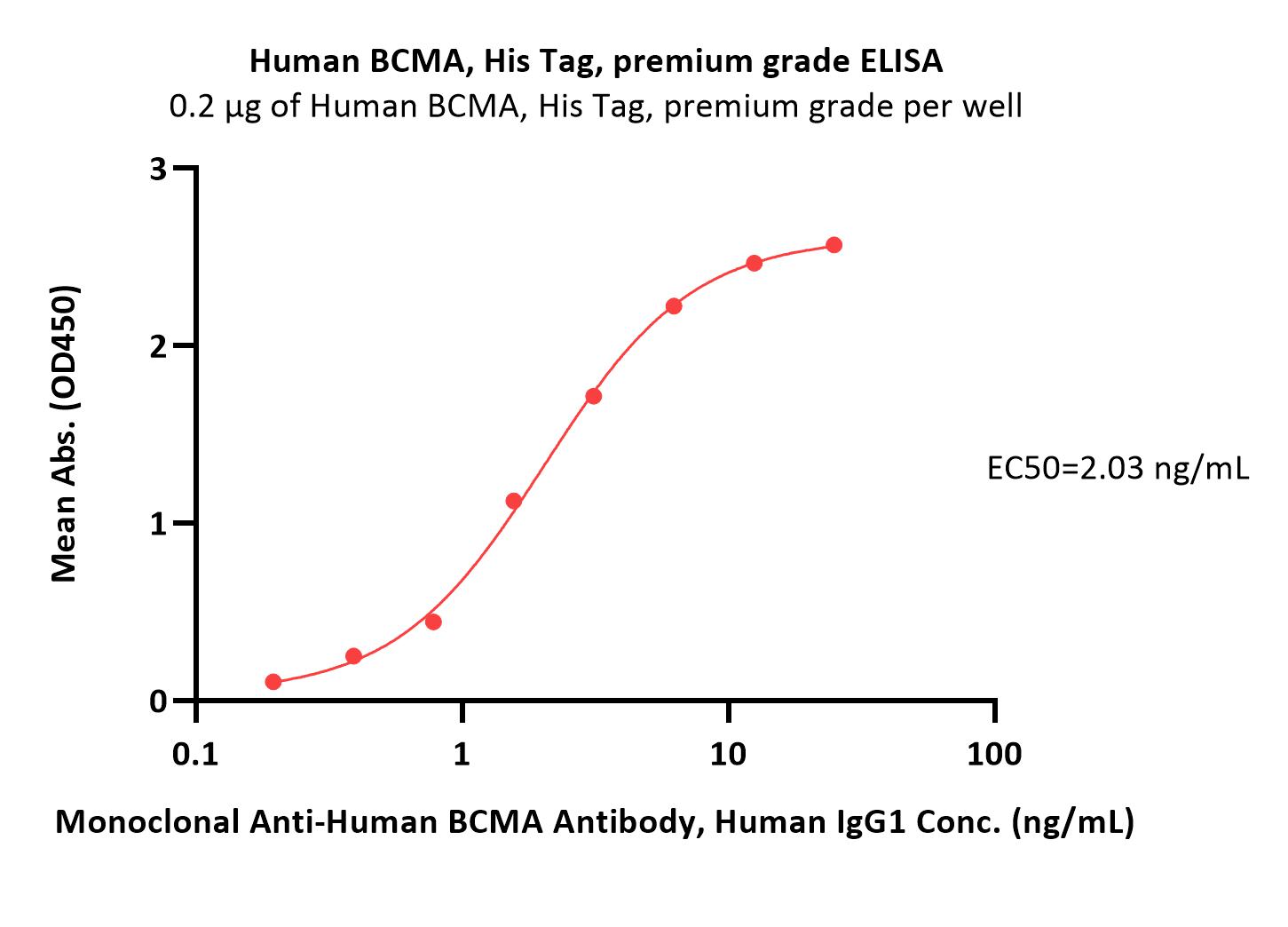
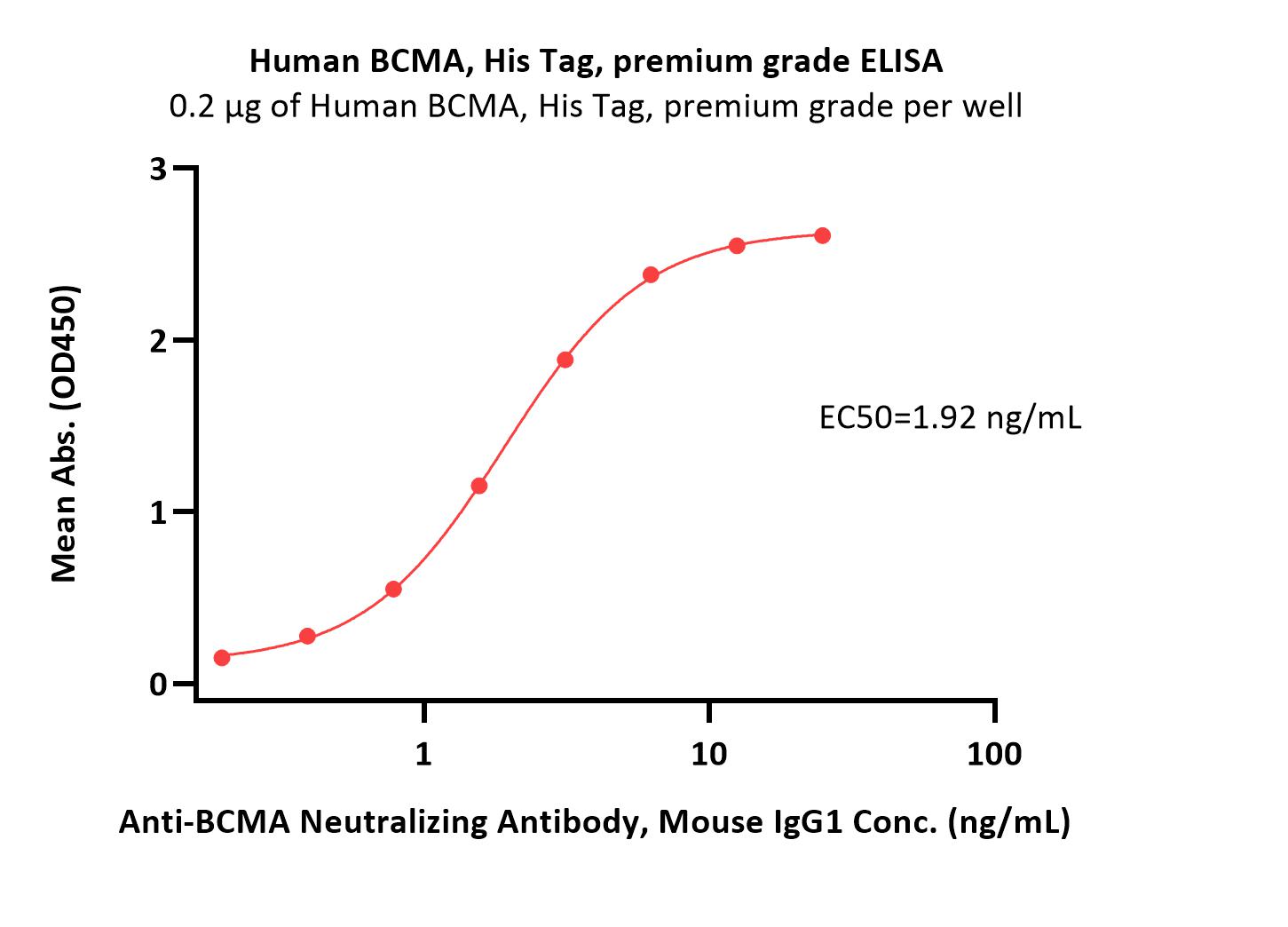
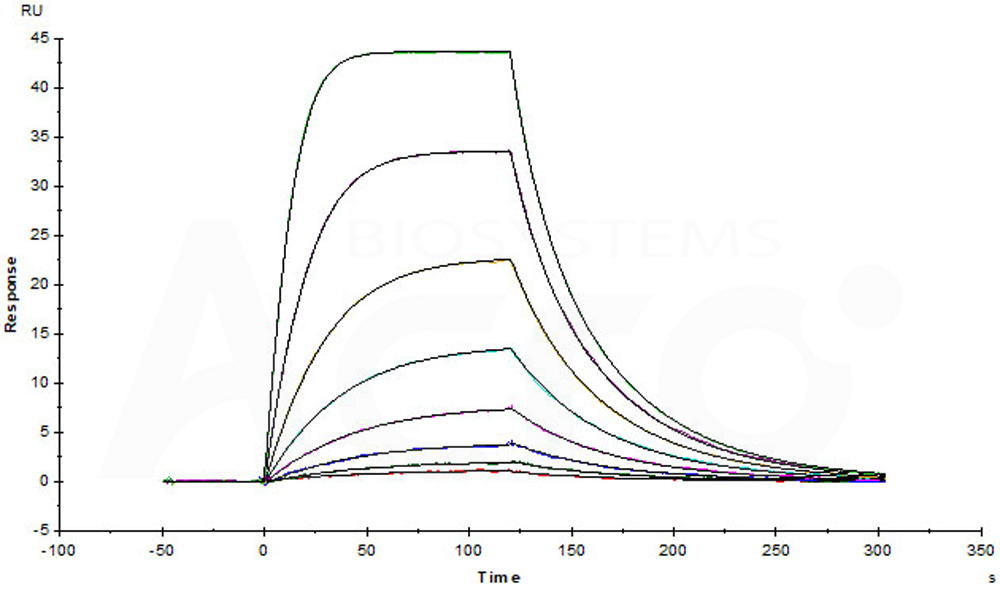
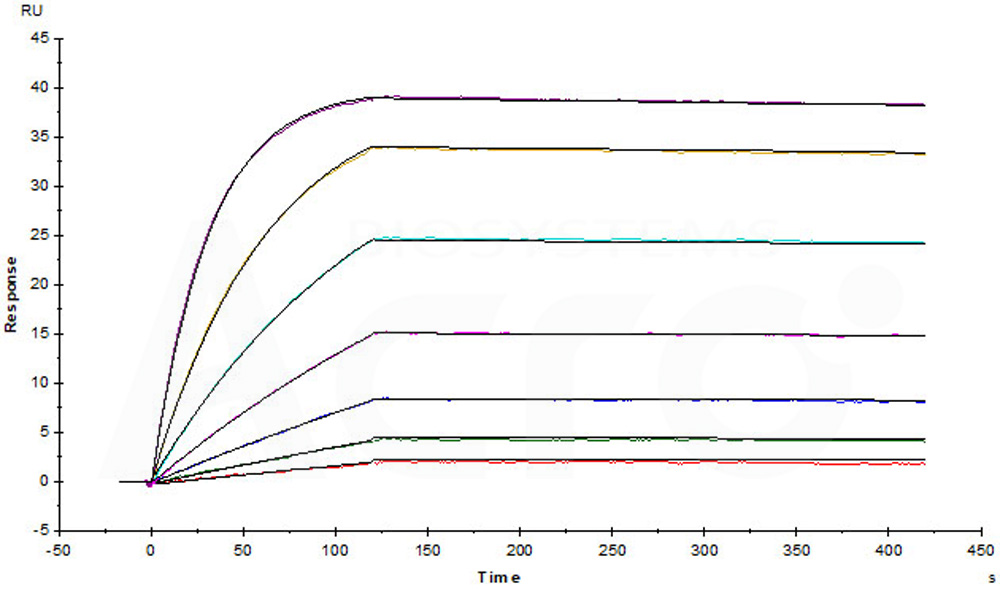
| Autologous BCMA-directed CAR T-cell therapy (The First Affiliated Hospital of Soochow University) |
|
Phase 3 Clinical |
The First Affiliated Hospital Of Soochow University |
Leukemia; Multiple Myeloma |
Details
|
| ABBV-383 |
TNB-383B; ABBV-383 |
Phase 3 Clinical |
Teneobio Inc, Abbvie Inc |
Multiple Myeloma |
Details
|
| Alnuctamab |
CC-93269; EM-901; BMS-986349 |
Phase 3 Clinical |
Engmab Ag |
Multiple Myeloma |
Details
|
| BCMA CAR-T Cells (Pregene) |
PRG-1801 |
Phase 2 Clinical |
Shenzhen Prekin Biopharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| Anitocabtagene autoleucel |
CART-ddBCMA; Kite-772 |
Phase 2 Clinical |
Arcellx Inc |
Multiple Myeloma |
Details
|
| Anti BCMA CAR T cell therapy (Shanghai Hrain Biotechnology) |
BCMA-CART; HR003 |
Phase 2 Clinical |
Hrain Biotechnology Co Ltd |
Multiple Myeloma |
Details
|
| Anti BCMA chimeric antigen receptor T cell therapy (Chongqing Precision Biotech) |
|
Phase 2 Clinical |
Chongqing Precision Biotechnology Co Ltd |
Lymphoma, B-Cell; Multiple Myeloma; Lymphoma, Non-Hodgkin |
Details
|
| BCMA CAR-T cell therapy (Chongqing Precision Biotech) |
|
Phase 2 Clinical |
Chongqing Precision Biotechnology Co Ltd |
Plasmacytoma; Multiple Myeloma; Neoplasms, Plasma Cell |
Details
|
| C-CAR088 |
C-CAR088 |
Phase 2 Clinical |
|
Multiple Myeloma |
Details
|
| Anti-BCMA chimeric antigen receptor T cell therapy (Cartesian) |
Descartes-08 |
Phase 2 Clinical |
Cartesian Therapeutics Inc |
Myasthenia Gravis; Multiple Myeloma; Lupus Erythematosus, Systemic |
Details
|
| Orvacabtagene autoleucel |
ET-140; FCARH-143; ET140-CAR; MCARH-171; JCARH-125 |
Phase 2 Clinical |
Juno Therapeutics Inc, Memorial Sloan Kettering Cancer Center, Eureka Therapeutics Inc |
Multiple Myeloma |
Details
|
| GC-012F |
GC-012F; GC012F |
Phase 2 Clinical |
Gracell Biotechnologies (Shanghai) Co Ltd |
Multiple Myeloma; Lupus Erythematosus, Systemic; Lymphoma, Non-Hodgkin |
Details
|
| BCMA CAR-NK 92 cell therapy (Asclepius Technology Company Group) |
|
Phase 2 Clinical |
Asclepius Technology Company Group |
Multiple Myeloma |
Details
|
| BCMA-PD1 CAR T cell therapy (General Hospital of the People's Liberation Army) |
|
Phase 2 Clinical |
People'S Liberation Army General Hospital Military Service |
Multiple Myeloma |
Details
|
| KQ-2003 |
KQ2003; KQ-2003 |
Phase 2 Clinical |
Shanghai Keqi Pharmaceutical Technology Co Ltd |
Solid tumours; Multiple Myeloma; POEMS Syndrome |
Details
|
| HDP-101 |
HDP-101; HDP101-ATAC |
Phase 2 Clinical |
Heidelberg |
Paraproteinemias; Multiple Myeloma |
Details
|
| REGN-5459 |
REGN-5459 |
Phase 2 Clinical |
|
Multiple Myeloma; Renal Insufficiency, Chronic |
Details
|
| Fourth-gen CAR T Cells Targeting BCMA/CD19 therapy(Essen Biotech) |
|
Phase 2 Clinical |
Essen Biotech |
Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Myositis; Sjogren's Syndrome; Scleroderma, Systemic; Autoimmune Diseases; Lupus Nephritis; Lupus Erythematosus, Systemic |
Details
|
| Anti-BCMA CAR-NK Therapy(Shahid Beheshti University of Medical Sciences) |
|
Phase 2 Clinical |
Shahid Beheshti University Of Medical Sciences |
Multiple Myeloma |
Details
|
| IBI-3003 |
IBI3003; IBI-3003 |
Phase 2 Clinical |
Innovent Biologics(Suzhou) Co Ltd |
Multiple Myeloma |
Details
|
| BCMA-GPRC5D CAR-T Cells Therapy(Wuhan Union Hospital) |
|
Phase 2 Clinical |
Guangzhou Bio-Gene Technology Co Ltd, Wuhan Union Hospital |
Multiple Myeloma |
Details
|
| CART-ASCT-CART2 cells Therapy(Institute Of Hematology & Blood Diseases Hospital) |
|
Phase 2 Clinical |
Institute Of Hematology & Blood Diseases Hospital |
Multiple Myeloma |
Details
|
| MBS-314 |
MBS-314 |
Phase 2 Clinical |
Beijing Mabworks Biotech Co Ltd |
Multiple Myeloma |
Details
|
| SAR445514 |
SAR445514; SAR-445514; IPH6401/SAR514 |
Phase 2 Clinical |
Sanofi |
Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma |
Details
|
| CART-BCMA(Simnova) |
SNC-102(Simnova); SNC102(Simnova) |
Phase 2 Clinical |
Shanghai Simnova Biotechnology Co Ltd |
Multiple Myeloma |
Details
|
| CAR-BCMA(Sheba Medical Center) |
|
Phase 2 Clinical |
Sheba Medical Center, Israel |
Multiple Myeloma |
Details
|
| Humanized CART Directed Against BCMA |
ARI-0002h |
Phase 2 Clinical |
Instituto De Salud Carlos Iii |
Multiple Myeloma |
Details
|
| Descartes-11 |
Descartes-11; Descartes-011 |
Phase 2 Clinical |
Cartesian Therapeutics Inc |
Multiple Myeloma |
Details
|
| BCMA CAR-T Cell Therapy (Shenzhen University General Hospital) |
|
Phase 2 Clinical |
Shenzhen University General Hospital |
Multiple Myeloma |
Details
|
| CM-336(Connaught Biomedical Technology) |
CM-336 |
Phase 2 Clinical |
Keymed Biosciences Co Ltd |
Multiple Myeloma |
Details
|
| PHE-885 |
PHE-885 |
Phase 2 Clinical |
Novartis Pharma Ag |
Hematologic Neoplasms; Multiple Myeloma |
Details
|
| ALLO-605 |
ALLO-605; ALLO605 |
Phase 2 Clinical |
Cellectis Sa |
Multiple Myeloma |
Details
|
| EMB-06 |
EMB-06; EMB06 |
Phase 2 Clinical |
Shanghai Epimab Biotherapeutics, Inc |
Multiple Myeloma |
Details
|
| NXC-201 |
HBI-0101; NXC-201 |
Phase 2 Clinical |
Immix Biopharma Inc, Hadassah Medical Organization |
Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma |
Details
|
| BCMA Targeted CAR-T Cell Therapy (Yake Biotechnology) |
|
Phase 2 Clinical |
Shanghai YaKe Biotechnology Co Ltd |
Neoplasms; Multiple Myeloma |
Details
|
| BCMA TriTAC |
HPN-217 |
Phase 1 Clinical |
Harpoon Therapeutics, Abbvie Inc |
Multiple Myeloma |
Details
|
| ACTR 087/SEA BCMA combination therapy |
|
Phase 1 Clinical |
Unum Therapeutics Inc |
Multiple Myeloma |
Details
|
| Motacabtagene lurevgedleucel |
CTX-120 |
Phase 1 Clinical |
Crispr Therapeutics Ag |
Multiple Myeloma |
Details
|
| AUTO-2 |
AUTO-2; SUB-96123 |
Phase 1 Clinical |
Autolus |
Multiple Myeloma |
Details
|
| Anti-CD19+/BCMA CAR-T cells (Hrain Biotechnology) |
|
Phase 1 Clinical |
Hrain Biotechnology Co Ltd |
POEMS Syndrome |
Details
|
| bb-21217 |
bb-21217 |
Phase 1 Clinical |
Bluebird Bio Inc, Bristol-Myers Squibb Company |
Multiple Myeloma |
Details
|
| AMG-224 |
AMG-224 |
Phase 1 Clinical |
Amgen Inc |
Multiple Myeloma |
Details
|
| Ispectamab debotansine |
CC-99712; BMS-986352; SP-8919 |
Phase 1 Clinical |
Celgene Corp |
Multiple Myeloma |
Details
|
| WVT-078(Novartis Pharma) |
WVT-078 |
Phase 1 Clinical |
Novartis Pharma Ag |
Multiple Myeloma |
Details
|
| Pacanalotamab |
AMG-420; BI-836909 |
Phase 1 Clinical |
Micromet Inc |
Multiple Myeloma |
Details
|
| ALLO-715 |
ALLO-715 |
Phase 1 Clinical |
Cellectis Sa |
Multiple Myeloma |
Details
|
| BCMA/CD19 CAR-T cell therapy (The First Affiliated Hospital of Nanchang University) |
|
Phase 1 Clinical |
The First Affiliated Hospital Of Nanchang University |
Multiple Myeloma |
Details
|
| Pavurutamab |
AMG-701 |
Phase 1 Clinical |
Amgen Inc |
Multiple Myeloma |
Details
|
| CART-BCMA |
MTV-273; CART-BCMA |
Phase 1 Clinical |
University Of Pennsylvania |
Rejection of renal transplantation; Renal Insufficiency; Multiple Myeloma; Kidney Failure, Chronic |
Details
|
| BCMA-CD19 cCAR T cell therapy (iCell Gene Therapeutics) |
|
Phase 1 Clinical |
Icell Gene Therapeutics (Int'L) Ltd |
Multiple Myeloma; Lupus Erythematosus, Systemic; Waldenstrom Macroglobulinemia |
Details
|
| Anti-BCMA anti-CD38 bispecific chimeric antigen receptor T cell therapy (Shengyan Pharmaceutical Technology) |
|
Phase 1 Clinical |
Shengyan Pharmaceutical Technology, Wuhan Union Hospital |
Multiple Myeloma |
Details
|
| Descartes-15 |
Descartes-015; Descartes-15 |
Phase 1 Clinical |
Cartesian Therapeutics Inc |
Solid tumours; Multiple Myeloma; Autoimmune Diseases of the Nervous System |
Details
|
| ACLX-001 |
ACLX-001 |
Phase 1 Clinical |
Arcellx Inc |
Multiple Myeloma |
Details
|
| CB-011 |
CB-011 |
Phase 1 Clinical |
Caribou Biosciences Inc |
Multiple Myeloma |
Details
|
| FT-576 |
FT-576 |
Phase 1 Clinical |
University Of Minnesota, Fate Therapeutics Inc |
Bone Marrow Neoplasms; Multiple Myeloma |
Details
|
| P-BCMA-ALLO1 |
P-BCMA-ALLO1 |
Phase 1 Clinical |
Transposagen Biopharmaceuticals Inc, Janssen Biotech Inc, Poseida Therapeutics Inc |
Multiple Myeloma |
Details
|
| BCMA CAR-NK (Hrain Biotechnology) |
HR012 |
Phase 1 Clinical |
Hrain Biotechnology Co Ltd |
Leukemia, Plasma Cell; Multiple Myeloma |
Details
|
| CD19/BCMA Hi-TCR-T cell therapy(Wuhan Union Hospital) |
|
Phase 1 Clinical |
Wuhan Union Hospital |
Lupus Erythematosus, Systemic |
Details
|
| The autologous dual target BCMA/CD19-CAR-T cell therapy(Nanjing University School Of Medicine) |
FKC-288; FKC288 |
Phase 1 Clinical |
Nanjing University School Of Medicine |
Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Lupus Nephritis |
Details
|
| C-CAR168 CAR T-cell therapy (AbelZeta) |
C-CAR168 |
Phase 1 Clinical |
AbelZeta Pharma Inc |
Multiple Sclerosis, Relapsing-Remitting; Autoimmune Diseases; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Muscular Diseases |
Details
|
| BCMAxGPRC5D CAR-T therapy (Juno Therapeutics) |
BMS-986453 |
Phase 1 Clinical |
Juno Therapeutics Inc |
Multiple Myeloma |
Details
|
| SIM-0500 |
SIM-0500; SIM0500 |
Phase 1 Clinical |
Hainan Xiansheng Re Ming Pharmaceutical Co Ltd |
Bone Marrow Neoplasms; Multiple Myeloma |
Details
|
| Recombinant anti BCMA/CD3 bispecific antibody(Hualan Genetic Engineering) |
|
Phase 1 Clinical |
Hualan Genetic Engineering (Henan) Co Ltd |
Multiple Myeloma |
Details
|
| BCMA Targeted CAR-T Cell Therapy (Carbiogene Therapeutics) |
CBG-002 CAR-T |
Phase 1 Clinical |
Carbiogene Therapeutics Co Ltd |
Multiple Myeloma |
Details
|
| BCMA-CD19 CAR T cell therapy (Hunan Siweikang Pharma) |
SWK001; SWK-001 |
Phase 1 Clinical |
Hunan Siweikang Pharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR T (Nanjing Kati Medical Technology) |
|
Phase 1 Clinical |
Nanjing Kati Medical Technology Co Ltd |
Multiple Myeloma |
Details
|
| Belantamab |
GSK2857914; GSK-2857914 |
Phase 1 Clinical |
Glaxosmithkline Plc |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR T-cell therapy (Actavis/Eugia Pharma) |
|
Phase 1 Clinical |
Eugia Pharma Specialities Ltd, Actavis Inc |
Multiple Myeloma |
Details
|
| LUCAR-B68 cells Therapy(Nanjing Legend Biotech Co) |
|
Phase 1 Clinical |
|
Multiple Myeloma |
Details
|
| TQB-2934 |
TQB2934; TQB-2934 |
Phase 1 Clinical |
Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group |
Multiple Myeloma |
Details
|
| MCARH-125 |
MCARH-125 |
Phase 1 Clinical |
Memorial Sloan Kettering Cancer Center |
Multiple Myeloma |
Details
|
| ISB-2001 |
ISB-2001 |
Phase 1 Clinical |
Ichnos Sciences Sa |
Multiple Myeloma |
Details
|
| OriC-321 |
OriC-321; Ori-C-321 |
Phase 1 Clinical |
Zhejiang University, OriCell Therapeutics Co Ltd |
Multiple Myeloma |
Details
|
| HY-027 |
HY-027 |
Phase 1 Clinical |
Juventas Cell Therapy Ltd |
Multiple Myeloma |
Details
|
| MEDI-2228 |
MEDI-2228 |
Phase 1 Clinical |
Medimmune Llc |
Multiple Myeloma |
Details
|
| IBI-346 |
IBI-346 |
Phase 1 Clinical |
Innovent Biologics(Suzhou) Co Ltd |
Multiple Myeloma |
Details
|
| BCMA-NKE |
BCMA-NKE |
Phase 1 Clinical |
Bristol-Myers Squibb Company |
Multiple Myeloma |
Details
|
| GR-1803 |
GR-1803 |
Phase 1 Clinical |
Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| MCM-998 |
MCM-998; LXG-250 |
Phase 1 Clinical |
Novartis Pharma Ag |
Multiple Myeloma |
Details
|
| F-182112 |
F-182112; F182112 |
Phase 1 Clinical |
Shandong New Time Pharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| Anti-BCMA/CD19 CAR-T Therapy(University College London) |
|
Phase 1 Clinical |
University College London |
Multiple Myeloma |
Details
|
| Recombinant humanized anti-BCMA/CD3 bispecific antibody(New Time Pharmaceutical) |
|
Phase 1 Clinical |
Shandong New Time Pharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| CXCR4 modified anti-BCMA CAR T cell therapy (Sichuan University) |
|
Phase 1 Clinical |
Sichuan University |
Multiple Myeloma |
Details
|
| JWCAR129 |
JWCAR129; JWCAR-129 |
Phase 1 Clinical |
Suzhou Yaomingjunuo Biotechnology Co Ltd |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR-T cell therapy (Xinqiao Hospital) |
|
Phase 1 Clinical |
Chongqing Xinqiao Hospital |
Multiple Myeloma |
Details
|
| BCMA-targeted universal LCAR-BCX cell therapy (Nanjing Legend Biotech) |
LCAR-BCX |
Phase 1 Clinical |
|
Multiple Myeloma |
Details
|
| CD19/BCMA-targeted CAR-T Cell Therapy (Zhejiang University) |
|
Phase 1 Clinical |
Zhejiang University, Shanghai YaKe Biotechnology Co Ltd |
Nephritis; Sjogren's Syndrome; Scleroderma, Systemic; Autoimmune Diseases; Multiple Myeloma; Lupus Nephritis; Lupus Erythematosus, Systemic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| C-4-29 |
|
Phase 1 Clinical |
Chongqing Precision Biotechnology Co Ltd |
Carcinoma, Renal Cell; Multiple Myeloma |
Details
|
| Anti-BCMA CAR-T cell therapy (PersonGen Biomedicine) |
|
Phase 1 Clinical |
Persongen Biotherapeutics |
Multiple Myeloma |
Details
|
| spCART-269 |
spCART-269 |
Phase 1 Clinical |
Shanghai Tongji Hospital, Tongji University School Of Medicine |
Multiple Myeloma |
Details
|
| BCMA CAR-T cell therapy (Hebei Senlang Biotechnology) |
|
Phase 1 Clinical |
Hebei Senlang Biological Technology Co Ltd |
Multiple Myeloma |
Details
|
| CC-98633 |
CC-98633 |
Phase 1 Clinical |
Celgene Corp |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR T cell therapy (Wuhan Bio-Raid Biotechnology) |
|
Clinical |
Wuhan BioRaid Biotechnology Co Ltd |
Hematologic Neoplasms |
Details
|
| Anti-BCMA CAR T-cell therapy (Huazhong University of Science and Technology) |
|
Clinical |
Huazhong University Of Science And Technology |
Multiple Myeloma |
Details
|
| PD-1Ab21-BCMACAR-T (CD biopharma) |
CD-003; CD-203 |
Clinical |
|
Multiple Myeloma |
Details
|
| CT-0590(The First Affiliated Hospital Of Soochow University) |
CT-0590 |
Clinical |
CARsgen Therapeutics Holdings Ltd |
Multiple Myeloma |
Details
|
| BCMA-UCART cell therapy (Shanghai Bioray Laboratory) |
|
Clinical |
BRL Medicine Inc |
Multiple Myeloma |
Details
|
| BCMA targeting UCAR-T cell therapy (PersonGen BioTherapeutics) |
UTAA17 |
|
Persongen Biotherapeutics (Suzhou) Co Ltd |
|
Details
|
| Autologous BCMA-directed CAR T-cell therapy (The First Affiliated Hospital of Soochow University) |
|
Phase 3 Clinical |
The First Affiliated Hospital Of Soochow University |
Leukemia; Multiple Myeloma |
Details
|
| ABBV-383 |
TNB-383B; ABBV-383 |
Phase 3 Clinical |
Teneobio Inc, Abbvie Inc |
Multiple Myeloma |
Details
|
| Alnuctamab |
CC-93269; EM-901; BMS-986349 |
Phase 3 Clinical |
Engmab Ag |
Multiple Myeloma |
Details
|
| BCMA CAR-T Cells (Pregene) |
PRG-1801 |
Phase 2 Clinical |
Shenzhen Prekin Biopharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| Anitocabtagene autoleucel |
CART-ddBCMA; Kite-772 |
Phase 2 Clinical |
Arcellx Inc |
Multiple Myeloma |
Details
|
| Anti BCMA CAR T cell therapy (Shanghai Hrain Biotechnology) |
BCMA-CART; HR003 |
Phase 2 Clinical |
Hrain Biotechnology Co Ltd |
Multiple Myeloma |
Details
|
| Anti BCMA chimeric antigen receptor T cell therapy (Chongqing Precision Biotech) |
|
Phase 2 Clinical |
Chongqing Precision Biotechnology Co Ltd |
Lymphoma, B-Cell; Multiple Myeloma; Lymphoma, Non-Hodgkin |
Details
|
| BCMA CAR-T cell therapy (Chongqing Precision Biotech) |
|
Phase 2 Clinical |
Chongqing Precision Biotechnology Co Ltd |
Plasmacytoma; Multiple Myeloma; Neoplasms, Plasma Cell |
Details
|
| C-CAR088 |
C-CAR088 |
Phase 2 Clinical |
|
Multiple Myeloma |
Details
|
| Anti-BCMA chimeric antigen receptor T cell therapy (Cartesian) |
Descartes-08 |
Phase 2 Clinical |
Cartesian Therapeutics Inc |
Myasthenia Gravis; Multiple Myeloma; Lupus Erythematosus, Systemic |
Details
|
| Orvacabtagene autoleucel |
ET-140; FCARH-143; ET140-CAR; MCARH-171; JCARH-125 |
Phase 2 Clinical |
Juno Therapeutics Inc, Memorial Sloan Kettering Cancer Center, Eureka Therapeutics Inc |
Multiple Myeloma |
Details
|
| GC-012F |
GC-012F; GC012F |
Phase 2 Clinical |
Gracell Biotechnologies (Shanghai) Co Ltd |
Multiple Myeloma; Lupus Erythematosus, Systemic; Lymphoma, Non-Hodgkin |
Details
|
| BCMA CAR-NK 92 cell therapy (Asclepius Technology Company Group) |
|
Phase 2 Clinical |
Asclepius Technology Company Group |
Multiple Myeloma |
Details
|
| BCMA-PD1 CAR T cell therapy (General Hospital of the People's Liberation Army) |
|
Phase 2 Clinical |
People'S Liberation Army General Hospital Military Service |
Multiple Myeloma |
Details
|
| KQ-2003 |
KQ2003; KQ-2003 |
Phase 2 Clinical |
Shanghai Keqi Pharmaceutical Technology Co Ltd |
Solid tumours; Multiple Myeloma; POEMS Syndrome |
Details
|
| HDP-101 |
HDP-101; HDP101-ATAC |
Phase 2 Clinical |
Heidelberg |
Paraproteinemias; Multiple Myeloma |
Details
|
| REGN-5459 |
REGN-5459 |
Phase 2 Clinical |
|
Multiple Myeloma; Renal Insufficiency, Chronic |
Details
|
| Fourth-gen CAR T Cells Targeting BCMA/CD19 therapy(Essen Biotech) |
|
Phase 2 Clinical |
Essen Biotech |
Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Myositis; Sjogren's Syndrome; Scleroderma, Systemic; Autoimmune Diseases; Lupus Nephritis; Lupus Erythematosus, Systemic |
Details
|
| Anti-BCMA CAR-NK Therapy(Shahid Beheshti University of Medical Sciences) |
|
Phase 2 Clinical |
Shahid Beheshti University Of Medical Sciences |
Multiple Myeloma |
Details
|
| IBI-3003 |
IBI3003; IBI-3003 |
Phase 2 Clinical |
Innovent Biologics(Suzhou) Co Ltd |
Multiple Myeloma |
Details
|
| BCMA-GPRC5D CAR-T Cells Therapy(Wuhan Union Hospital) |
|
Phase 2 Clinical |
Guangzhou Bio-Gene Technology Co Ltd, Wuhan Union Hospital |
Multiple Myeloma |
Details
|
| CART-ASCT-CART2 cells Therapy(Institute Of Hematology & Blood Diseases Hospital) |
|
Phase 2 Clinical |
Institute Of Hematology & Blood Diseases Hospital |
Multiple Myeloma |
Details
|
| MBS-314 |
MBS-314 |
Phase 2 Clinical |
Beijing Mabworks Biotech Co Ltd |
Multiple Myeloma |
Details
|
| SAR445514 |
SAR445514; SAR-445514; IPH6401/SAR514 |
Phase 2 Clinical |
Sanofi |
Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma |
Details
|
| CART-BCMA(Simnova) |
SNC-102(Simnova); SNC102(Simnova) |
Phase 2 Clinical |
Shanghai Simnova Biotechnology Co Ltd |
Multiple Myeloma |
Details
|
| CAR-BCMA(Sheba Medical Center) |
|
Phase 2 Clinical |
Sheba Medical Center, Israel |
Multiple Myeloma |
Details
|
| Humanized CART Directed Against BCMA |
ARI-0002h |
Phase 2 Clinical |
Instituto De Salud Carlos Iii |
Multiple Myeloma |
Details
|
| Descartes-11 |
Descartes-11; Descartes-011 |
Phase 2 Clinical |
Cartesian Therapeutics Inc |
Multiple Myeloma |
Details
|
| BCMA CAR-T Cell Therapy (Shenzhen University General Hospital) |
|
Phase 2 Clinical |
Shenzhen University General Hospital |
Multiple Myeloma |
Details
|
| CM-336(Connaught Biomedical Technology) |
CM-336 |
Phase 2 Clinical |
Keymed Biosciences Co Ltd |
Multiple Myeloma |
Details
|
| PHE-885 |
PHE-885 |
Phase 2 Clinical |
Novartis Pharma Ag |
Hematologic Neoplasms; Multiple Myeloma |
Details
|
| ALLO-605 |
ALLO-605; ALLO605 |
Phase 2 Clinical |
Cellectis Sa |
Multiple Myeloma |
Details
|
| EMB-06 |
EMB-06; EMB06 |
Phase 2 Clinical |
Shanghai Epimab Biotherapeutics, Inc |
Multiple Myeloma |
Details
|
| NXC-201 |
HBI-0101; NXC-201 |
Phase 2 Clinical |
Immix Biopharma Inc, Hadassah Medical Organization |
Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma |
Details
|
| BCMA Targeted CAR-T Cell Therapy (Yake Biotechnology) |
|
Phase 2 Clinical |
Shanghai YaKe Biotechnology Co Ltd |
Neoplasms; Multiple Myeloma |
Details
|
| BCMA TriTAC |
HPN-217 |
Phase 1 Clinical |
Harpoon Therapeutics, Abbvie Inc |
Multiple Myeloma |
Details
|
| ACTR 087/SEA BCMA combination therapy |
|
Phase 1 Clinical |
Unum Therapeutics Inc |
Multiple Myeloma |
Details
|
| Motacabtagene lurevgedleucel |
CTX-120 |
Phase 1 Clinical |
Crispr Therapeutics Ag |
Multiple Myeloma |
Details
|
| AUTO-2 |
AUTO-2; SUB-96123 |
Phase 1 Clinical |
Autolus |
Multiple Myeloma |
Details
|
| Anti-CD19+/BCMA CAR-T cells (Hrain Biotechnology) |
|
Phase 1 Clinical |
Hrain Biotechnology Co Ltd |
POEMS Syndrome |
Details
|
| bb-21217 |
bb-21217 |
Phase 1 Clinical |
Bluebird Bio Inc, Bristol-Myers Squibb Company |
Multiple Myeloma |
Details
|
| AMG-224 |
AMG-224 |
Phase 1 Clinical |
Amgen Inc |
Multiple Myeloma |
Details
|
| Ispectamab debotansine |
CC-99712; BMS-986352; SP-8919 |
Phase 1 Clinical |
Celgene Corp |
Multiple Myeloma |
Details
|
| WVT-078(Novartis Pharma) |
WVT-078 |
Phase 1 Clinical |
Novartis Pharma Ag |
Multiple Myeloma |
Details
|
| Pacanalotamab |
AMG-420; BI-836909 |
Phase 1 Clinical |
Micromet Inc |
Multiple Myeloma |
Details
|
| ALLO-715 |
ALLO-715 |
Phase 1 Clinical |
Cellectis Sa |
Multiple Myeloma |
Details
|
| BCMA/CD19 CAR-T cell therapy (The First Affiliated Hospital of Nanchang University) |
|
Phase 1 Clinical |
The First Affiliated Hospital Of Nanchang University |
Multiple Myeloma |
Details
|
| Pavurutamab |
AMG-701 |
Phase 1 Clinical |
Amgen Inc |
Multiple Myeloma |
Details
|
| CART-BCMA |
MTV-273; CART-BCMA |
Phase 1 Clinical |
University Of Pennsylvania |
Rejection of renal transplantation; Renal Insufficiency; Multiple Myeloma; Kidney Failure, Chronic |
Details
|
| BCMA-CD19 cCAR T cell therapy (iCell Gene Therapeutics) |
|
Phase 1 Clinical |
Icell Gene Therapeutics (Int'L) Ltd |
Multiple Myeloma; Lupus Erythematosus, Systemic; Waldenstrom Macroglobulinemia |
Details
|
| Anti-BCMA anti-CD38 bispecific chimeric antigen receptor T cell therapy (Shengyan Pharmaceutical Technology) |
|
Phase 1 Clinical |
Shengyan Pharmaceutical Technology, Wuhan Union Hospital |
Multiple Myeloma |
Details
|
| Descartes-15 |
Descartes-015; Descartes-15 |
Phase 1 Clinical |
Cartesian Therapeutics Inc |
Solid tumours; Multiple Myeloma; Autoimmune Diseases of the Nervous System |
Details
|
| ACLX-001 |
ACLX-001 |
Phase 1 Clinical |
Arcellx Inc |
Multiple Myeloma |
Details
|
| CB-011 |
CB-011 |
Phase 1 Clinical |
Caribou Biosciences Inc |
Multiple Myeloma |
Details
|
| FT-576 |
FT-576 |
Phase 1 Clinical |
University Of Minnesota, Fate Therapeutics Inc |
Bone Marrow Neoplasms; Multiple Myeloma |
Details
|
| P-BCMA-ALLO1 |
P-BCMA-ALLO1 |
Phase 1 Clinical |
Transposagen Biopharmaceuticals Inc, Janssen Biotech Inc, Poseida Therapeutics Inc |
Multiple Myeloma |
Details
|
| BCMA CAR-NK (Hrain Biotechnology) |
HR012 |
Phase 1 Clinical |
Hrain Biotechnology Co Ltd |
Leukemia, Plasma Cell; Multiple Myeloma |
Details
|
| CD19/BCMA Hi-TCR-T cell therapy(Wuhan Union Hospital) |
|
Phase 1 Clinical |
Wuhan Union Hospital |
Lupus Erythematosus, Systemic |
Details
|
| The autologous dual target BCMA/CD19-CAR-T cell therapy(Nanjing University School Of Medicine) |
FKC-288; FKC288 |
Phase 1 Clinical |
Nanjing University School Of Medicine |
Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Lupus Nephritis |
Details
|
| C-CAR168 CAR T-cell therapy (AbelZeta) |
C-CAR168 |
Phase 1 Clinical |
AbelZeta Pharma Inc |
Multiple Sclerosis, Relapsing-Remitting; Autoimmune Diseases; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Muscular Diseases |
Details
|
| BCMAxGPRC5D CAR-T therapy (Juno Therapeutics) |
BMS-986453 |
Phase 1 Clinical |
Juno Therapeutics Inc |
Multiple Myeloma |
Details
|
| SIM-0500 |
SIM-0500; SIM0500 |
Phase 1 Clinical |
Hainan Xiansheng Re Ming Pharmaceutical Co Ltd |
Bone Marrow Neoplasms; Multiple Myeloma |
Details
|
| Recombinant anti BCMA/CD3 bispecific antibody(Hualan Genetic Engineering) |
|
Phase 1 Clinical |
Hualan Genetic Engineering (Henan) Co Ltd |
Multiple Myeloma |
Details
|
| BCMA Targeted CAR-T Cell Therapy (Carbiogene Therapeutics) |
CBG-002 CAR-T |
Phase 1 Clinical |
Carbiogene Therapeutics Co Ltd |
Multiple Myeloma |
Details
|
| BCMA-CD19 CAR T cell therapy (Hunan Siweikang Pharma) |
SWK001; SWK-001 |
Phase 1 Clinical |
Hunan Siweikang Pharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR T (Nanjing Kati Medical Technology) |
|
Phase 1 Clinical |
Nanjing Kati Medical Technology Co Ltd |
Multiple Myeloma |
Details
|
| Belantamab |
GSK2857914; GSK-2857914 |
Phase 1 Clinical |
Glaxosmithkline Plc |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR T-cell therapy (Actavis/Eugia Pharma) |
|
Phase 1 Clinical |
Eugia Pharma Specialities Ltd, Actavis Inc |
Multiple Myeloma |
Details
|
| LUCAR-B68 cells Therapy(Nanjing Legend Biotech Co) |
|
Phase 1 Clinical |
|
Multiple Myeloma |
Details
|
| TQB-2934 |
TQB2934; TQB-2934 |
Phase 1 Clinical |
Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group |
Multiple Myeloma |
Details
|
| MCARH-125 |
MCARH-125 |
Phase 1 Clinical |
Memorial Sloan Kettering Cancer Center |
Multiple Myeloma |
Details
|
| ISB-2001 |
ISB-2001 |
Phase 1 Clinical |
Ichnos Sciences Sa |
Multiple Myeloma |
Details
|
| OriC-321 |
OriC-321; Ori-C-321 |
Phase 1 Clinical |
Zhejiang University, OriCell Therapeutics Co Ltd |
Multiple Myeloma |
Details
|
| HY-027 |
HY-027 |
Phase 1 Clinical |
Juventas Cell Therapy Ltd |
Multiple Myeloma |
Details
|
| MEDI-2228 |
MEDI-2228 |
Phase 1 Clinical |
Medimmune Llc |
Multiple Myeloma |
Details
|
| IBI-346 |
IBI-346 |
Phase 1 Clinical |
Innovent Biologics(Suzhou) Co Ltd |
Multiple Myeloma |
Details
|
| BCMA-NKE |
BCMA-NKE |
Phase 1 Clinical |
Bristol-Myers Squibb Company |
Multiple Myeloma |
Details
|
| GR-1803 |
GR-1803 |
Phase 1 Clinical |
Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| MCM-998 |
MCM-998; LXG-250 |
Phase 1 Clinical |
Novartis Pharma Ag |
Multiple Myeloma |
Details
|
| F-182112 |
F-182112; F182112 |
Phase 1 Clinical |
Shandong New Time Pharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| Anti-BCMA/CD19 CAR-T Therapy(University College London) |
|
Phase 1 Clinical |
University College London |
Multiple Myeloma |
Details
|
| Recombinant humanized anti-BCMA/CD3 bispecific antibody(New Time Pharmaceutical) |
|
Phase 1 Clinical |
Shandong New Time Pharmaceutical Co Ltd |
Multiple Myeloma |
Details
|
| CXCR4 modified anti-BCMA CAR T cell therapy (Sichuan University) |
|
Phase 1 Clinical |
Sichuan University |
Multiple Myeloma |
Details
|
| JWCAR129 |
JWCAR129; JWCAR-129 |
Phase 1 Clinical |
Suzhou Yaomingjunuo Biotechnology Co Ltd |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR-T cell therapy (Xinqiao Hospital) |
|
Phase 1 Clinical |
Chongqing Xinqiao Hospital |
Multiple Myeloma |
Details
|
| BCMA-targeted universal LCAR-BCX cell therapy (Nanjing Legend Biotech) |
LCAR-BCX |
Phase 1 Clinical |
|
Multiple Myeloma |
Details
|
| CD19/BCMA-targeted CAR-T Cell Therapy (Zhejiang University) |
|
Phase 1 Clinical |
Zhejiang University, Shanghai YaKe Biotechnology Co Ltd |
Nephritis; Sjogren's Syndrome; Scleroderma, Systemic; Autoimmune Diseases; Multiple Myeloma; Lupus Nephritis; Lupus Erythematosus, Systemic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| C-4-29 |
|
Phase 1 Clinical |
Chongqing Precision Biotechnology Co Ltd |
Carcinoma, Renal Cell; Multiple Myeloma |
Details
|
| Anti-BCMA CAR-T cell therapy (PersonGen Biomedicine) |
|
Phase 1 Clinical |
Persongen Biotherapeutics |
Multiple Myeloma |
Details
|
| spCART-269 |
spCART-269 |
Phase 1 Clinical |
Shanghai Tongji Hospital, Tongji University School Of Medicine |
Multiple Myeloma |
Details
|
| BCMA CAR-T cell therapy (Hebei Senlang Biotechnology) |
|
Phase 1 Clinical |
Hebei Senlang Biological Technology Co Ltd |
Multiple Myeloma |
Details
|
| CC-98633 |
CC-98633 |
Phase 1 Clinical |
Celgene Corp |
Multiple Myeloma |
Details
|
| Anti-BCMA CAR T cell therapy (Wuhan Bio-Raid Biotechnology) |
|
Clinical |
Wuhan BioRaid Biotechnology Co Ltd |
Hematologic Neoplasms |
Details
|
| Anti-BCMA CAR T-cell therapy (Huazhong University of Science and Technology) |
|
Clinical |
Huazhong University Of Science And Technology |
Multiple Myeloma |
Details
|
| PD-1Ab21-BCMACAR-T (CD biopharma) |
CD-003; CD-203 |
Clinical |
|
Multiple Myeloma |
Details
|
| CT-0590(The First Affiliated Hospital Of Soochow University) |
CT-0590 |
Clinical |
CARsgen Therapeutics Holdings Ltd |
Multiple Myeloma |
Details
|
| BCMA-UCART cell therapy (Shanghai Bioray Laboratory) |
|
Clinical |
BRL Medicine Inc |
Multiple Myeloma |
Details
|
| BCMA targeting UCAR-T cell therapy (PersonGen BioTherapeutics) |
UTAA17 |
|
Persongen Biotherapeutics (Suzhou) Co Ltd |
|
Details
|
 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !  Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!