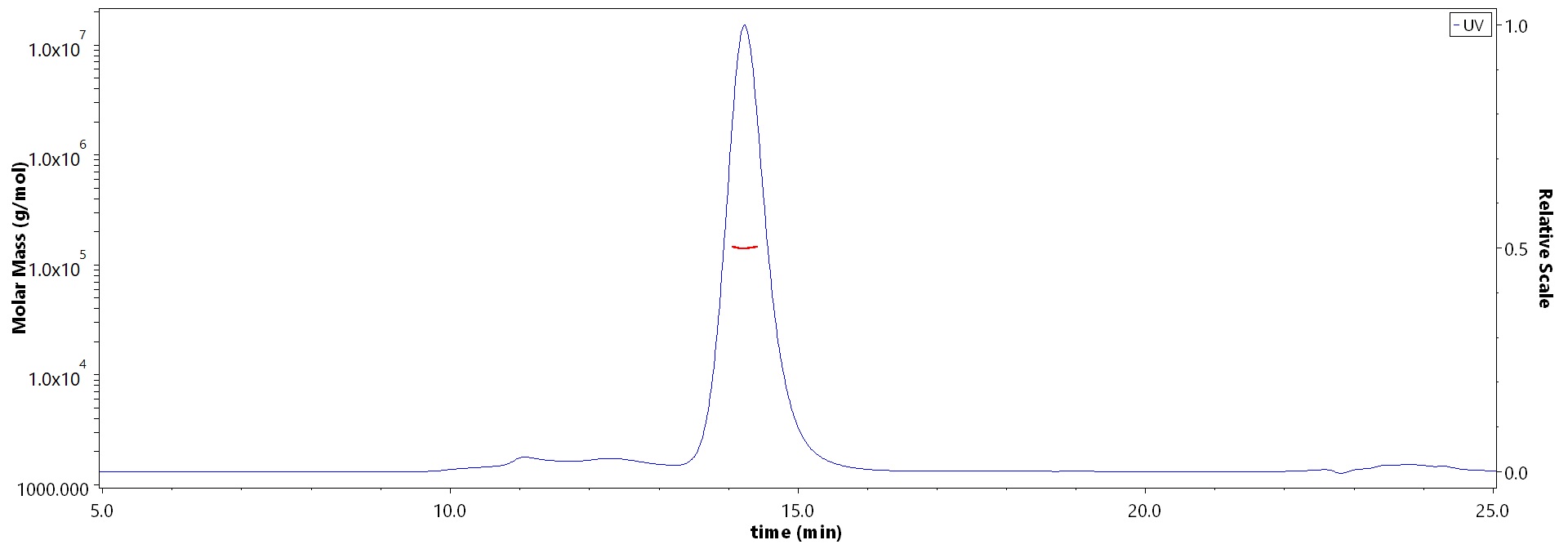
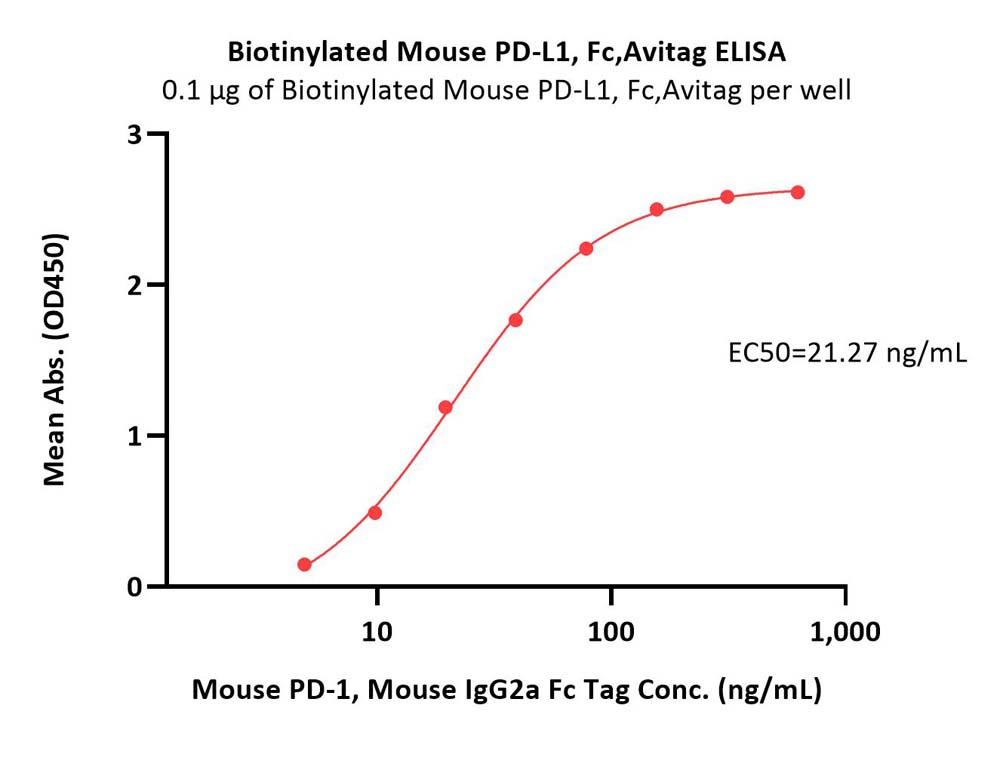
| Bintrafusp alfa |
M-7824; GSK-4045154; MSB-0011359C |
Phase 3 Clinical |
Merck Serono |
Lung Neoplasms; Sarcoma; Cholangiocarcinoma; Breast Neoplasms; Microsatellite Instability; Vulvar Neoplasms; Nasopharyngeal Carcinoma; Prostatic Neoplasms; Colorectal Neoplasms; Oropharyngeal Neoplasms; Thymus Neoplasms; Gallbladder Neoplasms; Adenomyoepithelioma; Lymphoma; Brain metastases; Urogenital Neoplasms; Thymoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Neoplasm Metastasis; Neoplasms, Germ Cell and Embryonal; Sarcoma, Kaposi; Triple Negative Breast Neoplasms; Solid tumours; Hematologic Neoplasms; Head and Neck Neoplasms; Intestinal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Anus Neoplasms; Stomach Neoplasms; Carcinoma; Carcinoma, Renal Cell; Esthesioneuroblastoma, Olfactory; Biliary Tract Neoplasms; Neoplasms; Colonic Neoplasms; Small Cell Lung Carcinoma; Papillomavirus Infections; Carcinoma, Transitional Cell; Nose Neoplasms; Ganglioglioma; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms |
Details
|
| Retlirafusp alfa |
SHR-1701 |
Phase 3 Clinical |
Jiangsu Hengrui Medicine Co Ltd |
Solid tumours; Esophageal Neoplasms; Rectal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Hodgkin Disease; Breast Neoplasms; Nasopharyngeal Carcinoma; Prostatic Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms |
Details
|
| Erfonrilimab |
KN046; KN-046 |
Phase 3 Clinical |
Jiangsu Alphamab Biopharmaceuticals Co Ltd |
Colorectal Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Thymoma; Esophageal Squamous Cell Carcinoma; Lymphoma; Solid tumours; Thymus Neoplasms; Breast Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Biliary Tract Neoplasms |
Details
|
| PM-8002 |
PM8002; PM-8002; BNT-327 |
Phase 3 Clinical |
Biotheus Inc |
Solid tumours; Triple Negative Breast Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Mesothelioma; Neuroendocrine Tumors; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung |
Details
|
| PD-L1 peptide vaccine (IO Biotech) |
IO-103 |
Phase 2 Clinical |
Herlev Hospital, Io Biotech Aps |
Carcinoma, Basal Cell; Smoldering Multiple Myeloma |
Details
|
| FAZ-053 |
FAZ-053; LAE-005; LAE005 |
Phase 2 Clinical |
Novartis Pharma Ag |
Solid tumours; Triple Negative Breast Neoplasms; Chordoma; Sarcoma, Alveolar Soft Part |
Details
|
| MAX-10181 |
MAX-10181; MAX-1 |
Phase 2 Clinical |
Maxinovel Pharmaceuticals Co Ltd |
Solid tumours; Neoplasms |
Details
|
| PD-L1 t-haNK cell therapy |
|
Phase 2 Clinical |
Nantkwest Inc |
Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Triple Negative Breast Neoplasms; Glioblastoma; Neoplasm Metastasis |
Details
|
| RC-98 |
RC-98; RC98 |
Phase 2 Clinical |
RemeGen Co Ltd |
Solid tumours; Stomach Neoplasms; Neoplasms |
Details
|
| Durvalumab/Gefitinib |
|
Phase 2 Clinical |
Medimmune |
Carcinoma, Non-Small-Cell Lung |
Details
|
| INCB-086550 |
INCB-086550; INCB-86550 |
Phase 2 Clinical |
Incyte Corp |
Solid tumours; Carcinoma; Carcinoma, Renal Cell; Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular |
Details
|
| BMS-936559 |
MDX-1105; BMS-936559 |
Phase 2 Clinical |
Bristol-Myers Squibb Company |
Leukemia, Myelogenous, Chronic; Shock, Septic; HIV Infections; Hodgkin Disease; Sepsis; Neoplasms; Small Cell Lung Carcinoma; Multiple Myeloma; Lymphoma, Non-Hodgkin; Melanoma |
Details
|
| PD-L1/CTLA4 bispecific monoclonal antibody (Changhai Hospital) |
|
Phase 2 Clinical |
Changhai Hospital Of Shanghai |
Pancreatic Neoplasms |
Details
|
| PM-8001 |
6MW3111; PM-8001 |
Phase 2 Clinical |
Biotheus Inc |
Solid tumours; Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| 89Zr-durvalumab |
|
Phase 2 Clinical |
Radboud University Nijmegen |
Head and Neck Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| Danburstotug |
STI-3031; STI-A1015; IMC-001 |
Phase 2 Clinical |
Sorrento Therapeutics Inc |
Solid tumours; Lymphoma, T-Cell, Peripheral; Biliary Tract Neoplasms; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Lymphoma; Bacterial Infections; Neoplasm Metastasis |
Details
|
| Recombinant humanized PD-L1 monoclonal antibody (Taizhou Houde Aoke Technology) |
LP-002 |
Phase 2 Clinical |
Taizhou Houde Aoke Technology Co Ltd |
Solid tumours; Lymphoma, B-Cell; Small Cell Lung Carcinoma; Digestive System Neoplasms; Primary mediastinal B cell lymphoma; Carcinoma, Squamous Cell; Melanoma |
Details
|
| Enristomig |
ES101; INBRX-105 |
Phase 2 Clinical |
Inhibrx |
Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Stomach Neoplasms; Thoracic Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Nasopharyngeal Carcinoma; Oropharyngeal Neoplasms; Esophageal adenocarcinoma; Neoplasm Metastasis; Melanoma; Carcinoma, Non-Small-Cell Lung |
Details
|
| AP-203 |
AP-203; AP203 |
Phase 2 Clinical |
AP Biosciences Inc |
Solid tumours; Squamous Cell Carcinoma of Head and Neck; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung |
Details
|
| HB-0036 |
HB-0036 |
Phase 2 Clinical |
|
Solid tumours; Carcinoma, Non-Small-Cell Lung |
Details
|
| FS 118 |
FS-118 |
Phase 2 Clinical |
Merck Serono, F-Star |
Squamous Cell Carcinoma of Head and Neck; Neoplasms; Neoplasm Metastasis |
Details
|
| Acasunlimab |
PD-L1x4-1BB; DuoBody-PD-L1x4-1BB; GEN1046; BNT-311 |
Phase 2 Clinical |
Genmab A/S, Biontech Se |
Solid tumours; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms |
Details
|
| JSKN-033 |
JSKN033; JSKN-033; JSKN-003/Envafolimab |
Phase 2 Clinical |
Jiangsu Alphamab Biopharmaceuticals Co Ltd |
Solid tumours |
Details
|
| MAX-80391 |
MAX-80391; MAX-8 |
Phase 2 Clinical |
Maxinovel Pharmaceuticals Co Ltd |
Solid tumours |
Details
|
| PRS-344 |
PRS-344/ONC0055; PRS-344; PRS-344/S095012; PRS-344S095012; S-095012 |
Phase 2 Clinical |
Pieris Pharmaceuticals Inc, Laboratoires Servier |
Solid tumours |
Details
|
| Pacmilimab |
CX-072 |
Phase 2 Clinical |
Cytomx Therapeutics Inc |
Solid tumours; Triple Negative Breast Neoplasms; Neoplasms; Breast Neoplasms; Lymphoma; Melanoma |
Details
|
| BPB-101 |
BPB-101 |
Phase 2 Clinical |
Betta Pharmaceuticals Co Ltd |
Solid tumours |
Details
|
| LB-101 (Centessa Pharmaceuticals) |
LB-101 (Centessa Pharmaceuticals); LB-101 Tetravalent PD-L1xCD47 LockBody bispecific monoclonal antibody |
Phase 2 Clinical |
Centessa Pharmaceuticals Plc |
Solid tumours |
Details
|
| AB-001 (Agastiya Biotech) |
AB01 (Agastiya Biotech); AB001 (Agastiya Biotech); AB-01 (Agastiya Biotech) |
Phase 2 Clinical |
Agastiya Biotech LLC |
Neoplasms |
Details
|
| 6MW-3511 |
6MW-3511 |
Phase 2 Clinical |
Mabwell (Shanghai) Bioscience Co Ltd |
Solid tumours; Neoplasms |
Details
|
| 18F-BMS-986229 |
18F-BMS-986229; [18F]BMS-986229 |
Phase 2 Clinical |
Bristol-Myers Squibb International Corp |
Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma |
Details
|
| sirpiglenastat |
DRP-104 |
Phase 2 Clinical |
Dracen Pharmaceuticals Inc |
Solid tumours; Fibrolamellar hepatocellular carcinoma; Carcinoma, Non-Small-Cell Lung |
Details
|
| Emfizatamab |
GNC-038 |
Phase 2 Clinical |
SystImmune |
Solid tumours; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Central Nervous System Lymphoma |
Details
|
| 99mTc-NM-01 |
99mTc-NM-01 |
Phase 2 Clinical |
Nanomab Technology Ltd |
Carcinoma, Non-Small-Cell Lung |
Details
|
| HB-0028 |
HB-0028 |
Phase 2 Clinical |
Shanghai Huaota Biopharmaceutical Co Ltd |
Solid tumours; Uterine Cervical Neoplasms |
Details
|
| PM-1022 |
PM-1022 |
Phase 2 Clinical |
Biotheus Inc |
Neoplasms |
Details
|
| LBL-024 |
LBL-024; LBL024 |
Phase 2 Clinical |
Nanjing Leads Biolabs Co Ltd |
Solid tumours; Neoplasms; Carcinoma, Neuroendocrine |
Details
|
| IBC-0966 |
IBC-0966 |
Phase 2 Clinical |
SunHo (China) BioPharmaceutical Co Ltd |
Neoplasms |
Details
|
| QLF31907 |
QLF31907; QLF-31907 |
Phase 2 Clinical |
Qilu Pharmaceutical Co Ltd |
Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Lymphoma, Non-Hodgkin; Melanoma |
Details
|
| INCB-099280 |
INCB-099280; INCB-99280 |
Phase 2 Clinical |
Incyte Corp Ltd |
Nasopharyngeal Carcinoma; Carcinoma, Hepatocellular; Melanoma; Uterine Cervical Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Squamous Cell; DNA Repair-Deficiency Disorders; Colorectal Neoplasms; Microsatellite Instability; Solid tumours; Mesothelioma; Microsatellite instability-high cancer; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Neoplasms; Carcinoma, Merkel Cell; Carcinoma, Renal Cell |
Details
|
| Nebratamig |
GNC-035 |
Phase 2 Clinical |
|
Solid tumours; Hematologic Neoplasms; Breast Neoplasms; Metastatic breast cancer; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Carrizumab |
|
Phase 2 Clinical |
Jiangsu Hengrui Pharmaceutical Co Ltd |
Esophageal Neoplasms; Stomach Neoplasms; Adenocarcinoma |
Details
|
| Recombinant anti-PD-L1/TGF-β bispecific antibody |
Y101D; Y-101D |
Phase 2 Clinical |
Wuhan Yzy Biopharma Co Ltd |
Solid tumours; Pancreatic Neoplasms; Carcinoma, Hepatocellular; Adenocarcinoma |
Details
|
| 6MW-3211 |
6MW3211; 6MW-3211 |
Phase 2 Clinical |
Mabwell (Shanghai) Bioscience Co Ltd |
Carcinoma, Renal Cell; Myelodysplastic Syndromes; Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Lymphoma |
Details
|
| ADG-104 |
ADG-104; ADG104 |
Phase 2 Clinical |
Guilin Sanjin Pharmaceutical Co Ltd, Adagene Inc |
Neoplasms |
Details
|
| Q-1802 |
Q-1802 |
Phase 2 Clinical |
Qiyu Biotechnology (Shanghai) Co Ltd |
Solid tumours; Esophageal Neoplasms; Gastrointestinal Neoplasms |
Details
|
| HLX-301 |
HLX-301 |
Phase 2 Clinical |
Shanghai Henlius Biologics Co Ltd |
Solid tumours; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Lymphoma; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis |
Details
|
| TQB-2858 |
TQB-2858 |
Phase 2 Clinical |
Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group |
Neoplasms; Pancreatic Neoplasms; Nasopharyngeal Neoplasms; Sarcoma; Endometrial Neoplasms; Uterine Cervical Neoplasms |
Details
|
| Sotiburafusp alfa |
HB0025; HB-0025 |
Phase 2 Clinical |
Shanghai Huaota Biopharmaceutical Co Ltd |
Hematologic Neoplasms; Solid tumours; Kidney Neoplasms; Carcinoma, Renal Cell; Endometrial Neoplasms; Carcinoma, Hepatocellular |
Details
|
| PD-L1 antibody combined with CTLA-4 antibody (Shanghai Zhongshan Hospital) |
|
Phase 2 Clinical |
Shanghai Zhongshan Hospital |
Cholangiocarcinoma |
Details
|
| HL-301 |
HL-301 |
Phase 2 Clinical |
Hanlim Pharm Co Ltd |
Radiation Pneumonitis; Small Cell Lung Carcinoma; Lung Neoplasms |
Details
|
| IMM-2510 |
IMM-2510 |
Phase 2 Clinical |
ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd |
Solid tumours; Sarcoma |
Details
|
| PM-1003 |
PM1003 |
Phase 2 Clinical |
Biotheus Inc |
Solid tumours |
Details
|
| Lesabelimab |
BC-003 |
Phase 2 Clinical |
Guilin Sanjin Pharmaceutical Co Ltd, Adagene (Suzhou) Ltd, Dragonboat Biopharmaceutical, Baifan Biotechnology (Shanghai) Co Ltd |
Carcinoma, Renal Cell; Neoplasms; Urinary Bladder Neoplasms; Penile Neoplasms |
Details
|
| Emdifen |
|
Phase 1 Clinical |
Tianjin Chase Sun Pharmaceutical Co Ltd, Chinese Academy Of Medical Sciences |
Solid tumours |
Details
|
| 89Zr-DFO-Atezolizumab |
89Zr-DFO-Atezolizumab |
Phase 1 Clinical |
The University Of Texas Southwestern Medical Center |
Carcinoma, Renal Cell; Diagnostic agents |
Details
|
| Chimeric antigen receptor T-cell therapy (Hunan Zhaotai Yongren Biotech) |
Z-CTLs |
Phase 1 Clinical |
Hunan Zhaotai Yongren Biotech Co Ltd |
Carcinoma, Non-Small-Cell Lung |
Details
|
| Lodapolimab |
LY-3300054 |
Phase 1 Clinical |
Eli Lilly And Company |
Solid tumours; Skin Melanoma; Carcinoma, Renal Cell; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Neoplasms; Microsatellite instability-high cancer; Breast Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| SL-279252 |
TAK-252; SL-279252 |
Phase 1 Clinical |
Takeda Pharmaceutical Co Ltd, Shattuck Labs Inc |
Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Carcinoma, Non-Small-Cell Lung; Melanoma; Adenocarcinoma |
Details
|
| LOR-S03 |
CDX-527 |
Phase 1 Clinical |
Celldex Therapeutics |
Ovarian Neoplasms; Solid tumours; Liver Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Neoplasms; Microsatellite instability-high cancer; Urinary Bladder Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| MCLA-145 |
MCLA-145 |
Phase 1 Clinical |
Incyte Corp, Merus Nv |
Solid tumours; Lymphoma, B-Cell; Neoplasms |
Details
|
| CAR-T cell therapy (Timmune Biotech) |
|
Phase 1 Clinical |
Tianjin Timmune Biotech Inc |
Multiple Myeloma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| Opucolimab |
HLX-20 |
Phase 1 Clinical |
Shanghai Henlius Biotech Inc |
Solid tumours; Neoplasms |
Details
|
| INCB-099318 |
INCB-099318; INCB-99318 |
Phase 1 Clinical |
Incyte Corp |
Neoplasms; Uterine Cervical Neoplasms; Penile Neoplasms; Endometrial Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Squamous Cell; Nasopharyngeal Carcinoma; Mesothelioma; Microsatellite instability-high cancer; Solid tumours; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Carcinoma, Basal Cell; Carcinoma, Renal Cell; Anus Neoplasms; Carcinoma, Merkel Cell; Ovarian Neoplasms |
Details
|
| Anti-CD19 CAR-T cells therapy (Tianjin Mycure Medical Technology) |
|
Phase 1 Clinical |
Tianjin Mycure Medical Technology Co Ltd |
Precursor B-Cell Lymphoblastic Leukemia-Lymphoma |
Details
|
| RG-6084 |
RG-6084; RO-7191863 |
Phase 1 Clinical |
F. Hoffmann-La Roche Ltd |
Hepatitis B |
Details
|
| IMMH-010 |
IMMH-010 |
Phase 1 Clinical |
Tianjin Chase Sun Pharmaceutical Co Ltd |
Solid tumours; Neoplasms |
Details
|
| Betifisolimab |
MSB-2311 |
Phase 1 Clinical |
Mabspace Biomedicine (Suzhou) Co Ltd |
Solid tumours |
Details
|
| Simridarlimab |
IBI-322; IBI322 |
Phase 1 Clinical |
Innovent Biologics(Suzhou) Co Ltd |
Hematologic Neoplasms; Solid tumours; Bone Marrow Neoplasms; Neoplasms; Leukemia, Myeloid, Acute; Lymphoma; Lymphoma, T-Cell |
Details
|
| AB-101 (Arbutus Biopharma) |
AB-101 (Arbutus Biopharma) |
Phase 1 Clinical |
Arbutus Biopharma Corp |
Hepatitis B |
Details
|
| HLX-43 |
HLX-43; HLX43 |
Phase 1 Clinical |
Shanghai Henlius Biotech Inc |
Solid tumours |
Details
|
| SIM-0237 |
SIM-0237 |
Phase 1 Clinical |
Simcere Pharmaceutical Group Ltd |
Solid tumours; Urinary Bladder Neoplasms |
Details
|
| LP-008 |
LP-008 |
Phase 1 Clinical |
|
Solid tumours |
Details
|
| EMB-09 |
EMB-09 |
Phase 1 Clinical |
Shanghai Epimab Biotherapeutics, Inc |
Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Endometrial Neoplasms; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung |
Details
|
| CYTO-102 |
CYTO-NK-102; CYTO-102; COH06 |
Phase 1 Clinical |
CytoImmune Therapeutics Inc |
Lung Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| TS-1905 |
TS-1905; LY-01019; BA-1201 |
Phase 1 Clinical |
|
Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Papillomavirus Infections; Carcinoma, Non-Small-Cell Lung |
Details
|
| BH-3120 |
BH-3120 |
Phase 1 Clinical |
Hanmi Pharmaceutical Co Ltd |
Solid tumours |
Details
|
| Xirestomig |
ATG-101; ATG101; YN051 |
Phase 1 Clinical |
The Origin Cell Technology Group Co Ltd |
Solid tumours; Hematologic Neoplasms; Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Neoplasm Metastasis |
Details
|
| QL-301 |
QL-301 |
Phase 1 Clinical |
Qlsf Biotherapeutics Inc |
Neoplasms |
Details
|
| MVR-C5252 |
C-5252; MVR-C5252 |
Phase 1 Clinical |
Immvira Co Ltd |
Solid tumours; Ganglioglioma; Glioblastoma; Brain Neoplasms; Glioma |
Details
|
| Q-1801 |
Q-1801; Q 1801 |
Phase 1 Clinical |
Qiyu Biotechnology (Shanghai) Co Ltd |
Solid tumours |
Details
|
| IMGS-001 |
IMGS-001 |
Phase 1 Clinical |
The University Of Texas MD Anderson Cancer Center |
Solid tumours; Neoplasms; Triple Negative Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms |
Details
|
| ABL-501 |
ABL-501 |
Phase 1 Clinical |
Abl Bio Inc |
Solid tumours |
Details
|
| CTX-8371 |
CTX-8371 |
Phase 1 Clinical |
Compass Therapeutics LLC |
Solid tumours; Squamous Cell Carcinoma of Head and Neck; Hodgkin Disease; Triple Negative Breast Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung |
Details
|
| IBC-Ab002 |
IBC-Ab002 |
Phase 1 Clinical |
ImmunoBrain Checkpoint Inc |
Alzheimer Disease |
Details
|
| Pd-1-pik |
Pd-1-pik |
Phase 1 Clinical |
Huashan Hospital Affiliated To Fudan University |
Glioblastoma |
Details
|
| Reozalimab |
IBI-318; LY-3434172; LY3434172 |
Phase 1 Clinical |
Innovent Biologics(Suzhou) Co Ltd, Eli Lilly And Company |
Neoplasms; Small Cell Lung Carcinoma; Lymphoma, Extranodal NK-T-Cell; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular |
Details
|
| LW-347 |
LW-347 |
Phase 1 Clinical |
Shanghai Longwood Biopharmaceuticals Co Ltd |
Neoplasms |
Details
|
| A-337 (Klus Pharma) |
A337 (Klus Pharma); A-337 (Klus Pharma) |
Phase 1 Clinical |
Klus Pharma Inc |
Neoplasms |
Details
|
| PMI-06 |
PMI06; PMI-06 |
Phase 1 Clinical |
Precision Molecular Inc |
Carcinoma, Non-Small-Cell Lung |
Details
|
| YL-222 |
YL222; YL-222 |
Phase 1 Clinical |
MediLink Therapeutics Co Ltd |
Solid tumours |
Details
|
| ND-021 |
ND-021; NM21-1480; CS-2006; CS2006 |
Phase 1 Clinical |
Numab Therapeutics Ag, Cstone Pharmaceuticals |
Solid tumours; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung |
Details
|
| SPX-303 |
SPX-303 |
Phase 1 Clinical |
SparX Biopharmaceutical Corp |
Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck |
Details
|
| KM602 |
KM602; KM-602; XZP-KM602 |
Phase 1 Clinical |
Xuanzhu (Shijiazhuang) Biotechnology Co Ltd |
Solid tumours |
Details
|
| B-901 |
B-901; B901 |
Phase 1 Clinical |
FutureGen Biopharm (Beijing) Co Ltd |
Solid tumours |
Details
|
| Recombinant anti-PD-L1/TGF-β bifunctional fusion protein |
|
Phase 1 Clinical |
Hualan Genetic Engineering (Henan) Co Ltd |
Solid tumours |
Details
|
| AUR-106 |
AUR-106 |
Phase 1 Clinical |
Aurigene Discovery Technologies Ltd |
Kidney Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| 89Zr-S095012 |
89Zr-S-095012 |
Phase 1 Clinical |
Institut De Recherches Internationales Servier |
Solid tumours |
Details
|
| RAD-204 |
RAD-204; 177Lu-RAD204 |
Phase 1 Clinical |
Radiopharm Theranostics Ltd |
Carcinoma, Non-Small-Cell Lung |
Details
|
| RAD-203 |
RAD-203 |
Phase 1 Clinical |
Radiopharm Theranostics Ltd |
Carcinoma, Non-Small-Cell Lung |
Details
|
| HK-010 |
HK-010; HK010 |
Phase 1 Clinical |
Anhui Anke Biotechnology (Group) Co Ltd, Hefei Hankemab Biotechnology Co Ltd |
Neoplasms |
Details
|
| Dual-targeting VEGFR1 and PD-L1 CAR-T cells Therapy (Sichuan University) |
|
Phase 1 Clinical |
Sichuan University |
Serositis; Ascites |
Details
|
| Ori-Bs-001 |
Ori-Bs-001 |
Phase 1 Clinical |
OriCell Therapeutics Co Ltd |
Solid tumours |
Details
|
| BC008-1A |
BC008-1A |
Phase 1 Clinical |
Sichuan Luzhou Buchang Biopharmaceutical Co Ltd |
Solid tumours |
Details
|
| B-1962 |
B1962; AP-505 |
Phase 1 Clinical |
AP Biosciences Inc |
Solid tumours; Liver Neoplasms; Neoplasms; Lung Neoplasms |
Details
|
| LB-1410 |
LB-1410 |
Phase 1 Clinical |
L&L Biopharma Co Ltd |
Solid tumours; Neoplasms; Lymphoma |
Details
|
| HBM-9027 |
HBM-9027; HBM9027 |
Phase 1 Clinical |
Harbour Biomed |
Solid tumours; Neoplasms |
Details
|
| QL-415 |
QL-415 |
Phase 1 Clinical |
Qlsf Biotherapeutics Inc |
Neoplasms |
Details
|
| SGN-PDL1V |
SGN-PDL1V |
Phase 1 Clinical |
|
Ovarian Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Melanoma |
Details
|
| ASC-61 |
ASC-61 |
Phase 1 Clinical |
Ascletis Pharma Inc |
Solid tumours; Neoplasms |
Details
|
| DR-30206 |
DR-30206 |
Phase 1 Clinical |
Zhejiang Doer Biologics Corp |
Solid tumours |
Details
|
| BPI-371153 |
BPI-371153 |
Phase 1 Clinical |
Betta Pharmaceuticals Co Ltd |
Solid tumours; Liver Neoplasms; Lymphoma; Carcinoma, Non-Small-Cell Lung |
Details
|
| CS-23546 |
CS-23546; CS23546 |
Phase 1 Clinical |
Shenzhen Chipscreen Biosciences Co Ltd |
Neoplasms; Immune System Diseases |
Details
|
| SG-1408 |
SG-1408; SG1408 |
Phase 1 Clinical |
Hangzhou Sumgen Biotechnology Co Ltd |
Solid tumours |
Details
|
| CHECKvacc |
CF33-hNIS-antiPDL1 |
Phase 1 Clinical |
Imugene Ltd, City Of Hope National Medical Center |
Triple Negative Breast Neoplasms; Breast Neoplasms; Metastatic breast cancer |
Details
|
| 706-3SBio |
SSGJ-706; 706 anti -PD1/PD -L1 BsAb; 706 anti-PD1/PD-L1 Bispecific antibody |
Phase 1 Clinical |
3sbio Inc |
Solid tumours; Composite Lymphoma; Lymphoma |
Details
|
| Gallium [68Ga] Natan recombinant PD-L1 single domain antibody |
SNA-002; 68Ga-NOTA-SNA002 |
Phase 1 Clinical |
Smartnuclide Biopharma |
Solid tumours; Neoplasms; Contrast agents |
Details
|
| SHS-009 |
SHS-009; SH009; SH-009 |
Phase 1 Clinical |
Nanjing Sanhome Pharmaceutical Co Ltd |
Neoplasms |
Details
|
| SHS-006(Nanjing Sanhome Pharmaceutical) |
SHS-006; SH-006; SH006 |
Phase 1 Clinical |
Nanjing Sanhome Pharmaceutical Co Ltd |
Solid tumours |
Details
|
| CN-202 |
CN-202 |
Phase 1 Clinical |
Curon Biopharmaceutical Ltd |
Hematologic Neoplasms; Solid tumours; Neoplasm Metastasis |
Details
|
| Recombinant oncolytic type II herpes simplex virus (Binhui Biopharmaceutical) |
BS-006; oHSV2-BiTEs |
Phase 1 Clinical |
Wuhan Binhui Biotechnology Co Ltd |
Solid tumours; Neoplasms; Melanoma; Uterine Cervical Neoplasms |
Details
|
| FS-222 |
FS-222 |
Phase 1 Clinical |
F-star Beta Ltd |
Neoplasms; Neoplasm Metastasis |
Details
|
| IMM-2520 |
IMM-2520 |
Phase 1 Clinical |
ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd |
Solid tumours; Squamous Cell Carcinoma of Head and Neck; Small Cell Lung Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| BAT-7104 |
BAT-7104 |
Phase 1 Clinical |
Bio-Thera Solutions Ltd |
Solid tumours; Neoplasms |
Details
|
| CA-170 |
CA-170; AUPM-170 |
Phase 1 Clinical |
Aurigene |
Solid tumours |
Details
|
| FH-2001 |
FH-2001 |
Phase 1 Clinical |
Shanghai Fosun Pharmaceutical Industrial Development Co Ltd |
Solid tumours |
Details
|
| TT-00420/Atezolizumab |
|
Phase 1 Clinical |
TransThera Sciences (Nanjing) Inc |
Gastrointestinal Neoplasms |
Details
|
| PF-07257876 |
|
Phase 1 Clinical |
Pfizer Inc |
Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Non-Small-Cell Lung |
Details
|
| AN-4005 |
AN-4005 |
Phase 1 Clinical |
Hangzhou Adlai Nortye Biopharma Co Ltd |
Solid tumours; Neoplasms; Lymphoma |
Details
|
| BR-102 |
BR102 |
Phase 1 Clinical |
BioRay Pharmaceutical Co Ltd |
Solid tumours; Neoplasms |
Details
|
| TST-005 |
TST-005; TST005 |
Phase 1 Clinical |
Mabspace Biomedicine (Suzhou) Co Ltd |
Solid tumours; Neoplasms; Papillomavirus Infections |
Details
|
| GT-90008 |
GS-19-PLB-1C; GT-90008; GS-19 |
Phase 1 Clinical |
Suzhou Koshine Biomedica Inc |
Solid tumours |
Details
|
| TJ-L14B |
ABL-503; TJ-L14B/ABL503; ABL503 |
Phase 1 Clinical |
I-Mab Biopharma Co Ltd |
Solid tumours |
Details
|
| CCX-559 |
CCX-559 |
Phase 1 Clinical |
Chemocentryx Inc |
Solid tumours; Neoplasms |
Details
|
| Dual-targeting HER2 and PD-L1 CAR-T Cell Therapy (Sichuan University) |
|
Phase 1 Clinical |
Sichuan University |
Peritoneal Neoplasms; Pleural Effusion, Malignant |
Details
|
| QLS31901 |
QLS-31901; QLS31901 |
Phase 1 Clinical |
Qilu Pharmaceutical Co Ltd |
Neoplasms |
Details
|
| IMM2505 |
IMM2505; IMM-2505 |
Phase 1 Clinical |
SunHo (China) BioPharmaceutical Co Ltd, ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd |
Solid tumours |
Details
|
| SKB-337 |
SKB337; A-337; SKB-337 |
Phase 1 Clinical |
Sichuan Kelun-Biotech Biopharmaceutical Co Ltd |
Solid tumours |
Details
|
| GNC-039 |
GNC-039 |
Phase 1 Clinical |
|
Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis |
Details
|
| ABSK-043 |
ABSK043 |
Phase 1 Clinical |
ABbisko Therapeutics Co Ltd |
Solid tumours; Neoplasms |
Details
|
| IBI-323(Innovent Biologics) |
IBI-323 |
Phase 1 Clinical |
Innovent Biologics(Suzhou) Co Ltd |
Solid tumours; Neoplasms |
Details
|
| KN-052 |
KN-052 |
Phase 1 Clinical |
Suzhou Alphamab Co Ltd |
Solid tumours |
Details
|
| SG12473 |
SG12473; SG-12473 |
Phase 1 Clinical |
Hangzhou Sumgen Biotechnology Co Ltd |
Hematologic Neoplasms; Solid tumours; Neoplasms |
Details
|
| BJ-005 |
BJ-005 |
Phase 1 Clinical |
Boji Biomedical Technology (Hangzhou) Co Ltd |
Solid tumours; Lymphoma |
Details
|
| MT-6402 |
MT-6402; MT-64-6402 |
Phase 1 Clinical |
Molecular Templates Inc |
Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Non-Small-Cell Lung |
Details
|
| FPT-155 |
CD80-Fc; FPT-155 |
Phase 1 Clinical |
Five Prime Therapeutics Inc |
Solid tumours |
Details
|
| Garivulimab |
BGB-A333 |
Phase 1 Clinical |
Beigene Ltd |
Solid tumours; Neoplasms |
Details
|
| Recombinant anti-PD-L1 human monoclonal antibody (Jiansu huaiyu) |
|
Phase 1 Clinical |
Jiangsu Huaiyu Pharmaceutical Co Ltd |
Solid tumours |
Details
|
| Durvalumab/Selumetinib sulfate |
|
Phase 1 Clinical |
Astrazeneca Plc |
Neoplasms |
Details
|
| 18F-BMS-986192 |
BMS-986192-[18F] |
Clinical |
Bristol-Myers Squibb Company |
Neoplasms |
Details
|
| 89Zr-KN035(Wuxi No. 4 People's Hospital) |
|
Clinical |
Wuxi No. 4 People |
Solid tumours |
Details
|
 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !  Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!