 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| RAS-SP006 | Mouse | Mouse IL-2 ELISPOT Kit | |||
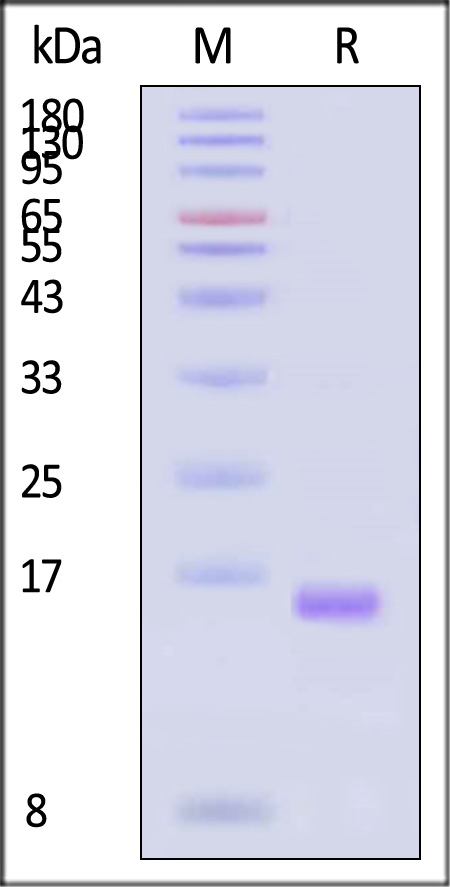
| GMP-L02H14 | Human | GMP Human IL-2 Protein |  |
|
|
| CRS-B008 | Human | ClinMax™ Human IL-2 ELISA Kit | |||
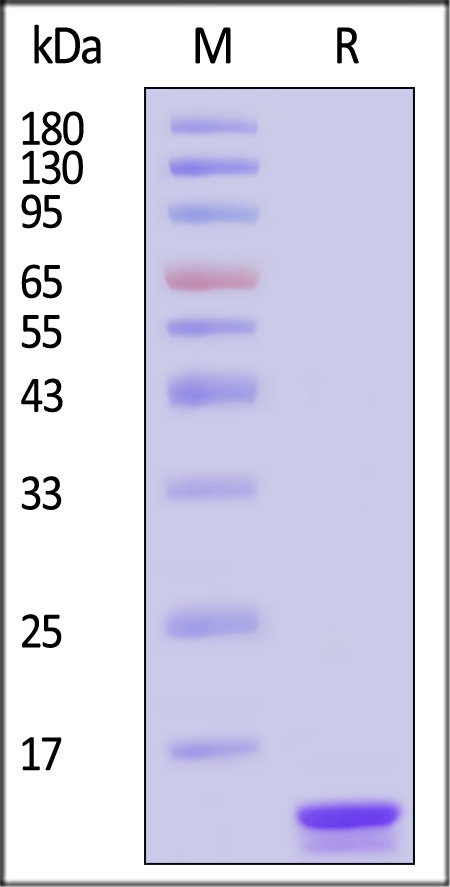
| IL2-H5215 | Human | Human IL-2 Protein, Tag Free (MALS verified) |  |
|
|
| CRS-A003 | Human | resDetect™ Human Interleukin-2 (IL-2) ELISA Kit (Residue Testing) | |||
| IL2-C5249 | Cynomolgus | Cynomolgus IL-2 Protein, His Tag |  |

|
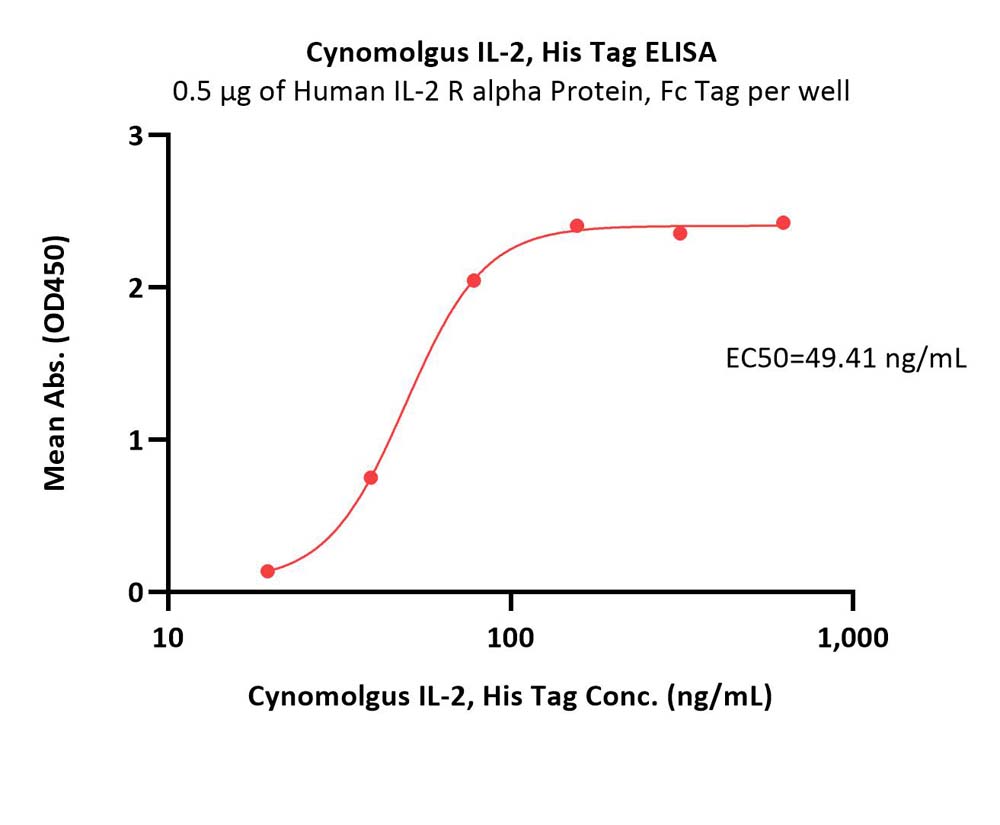

|
| IL2-H5269 | Human | Human IL-2 Protein, Fc Tag (MALS verified) |  |

|

|
| IL2-M52H3 | Mouse | Mouse IL-2 Protein, His Tag | 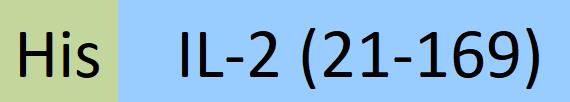 |

|
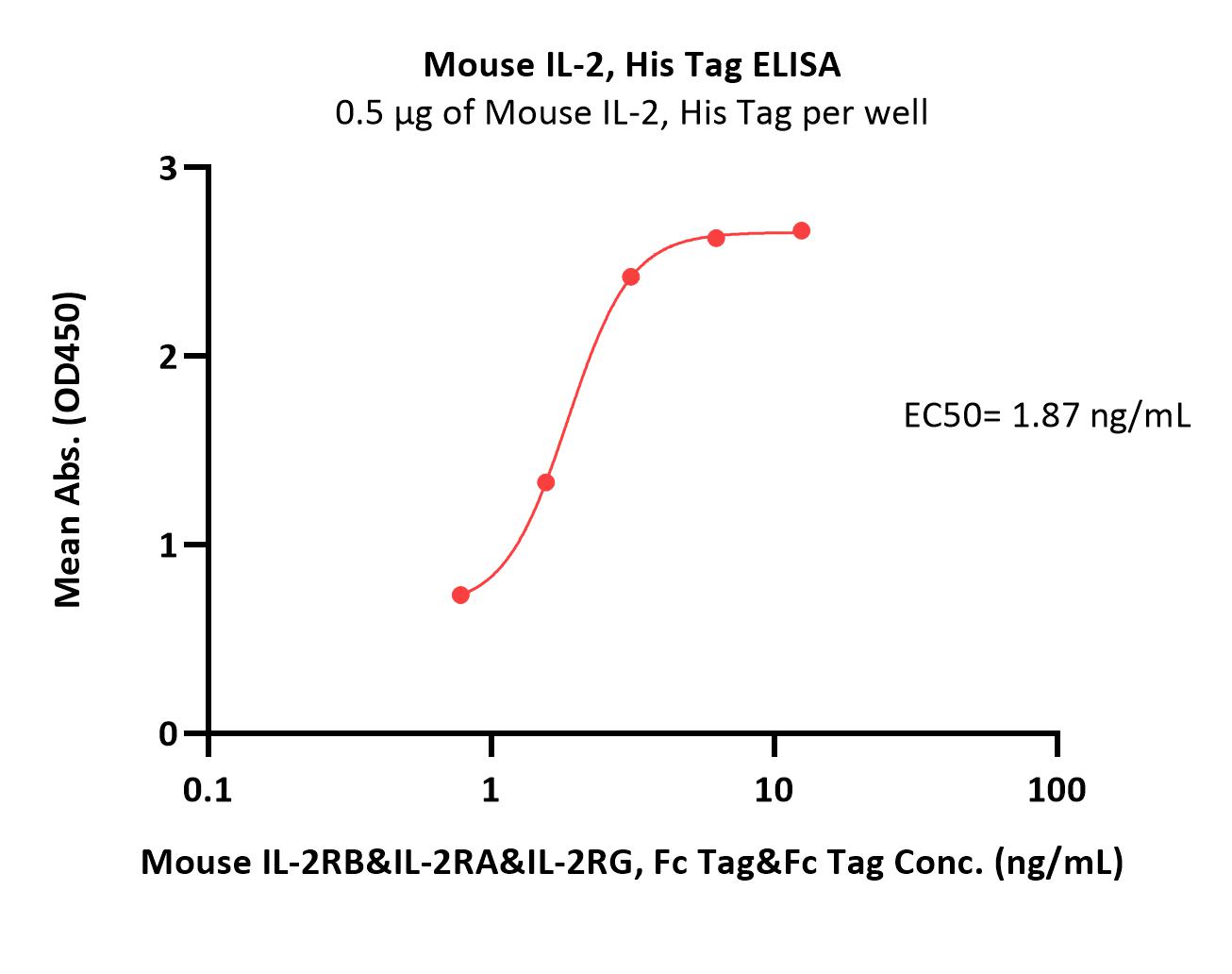
|
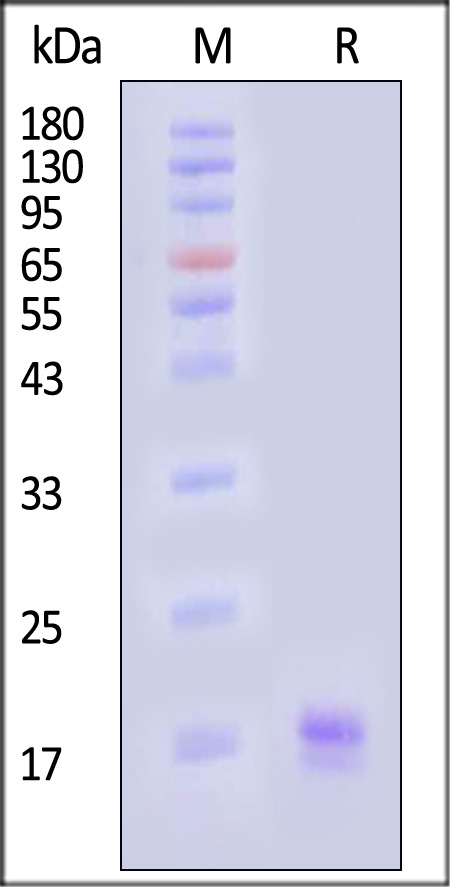
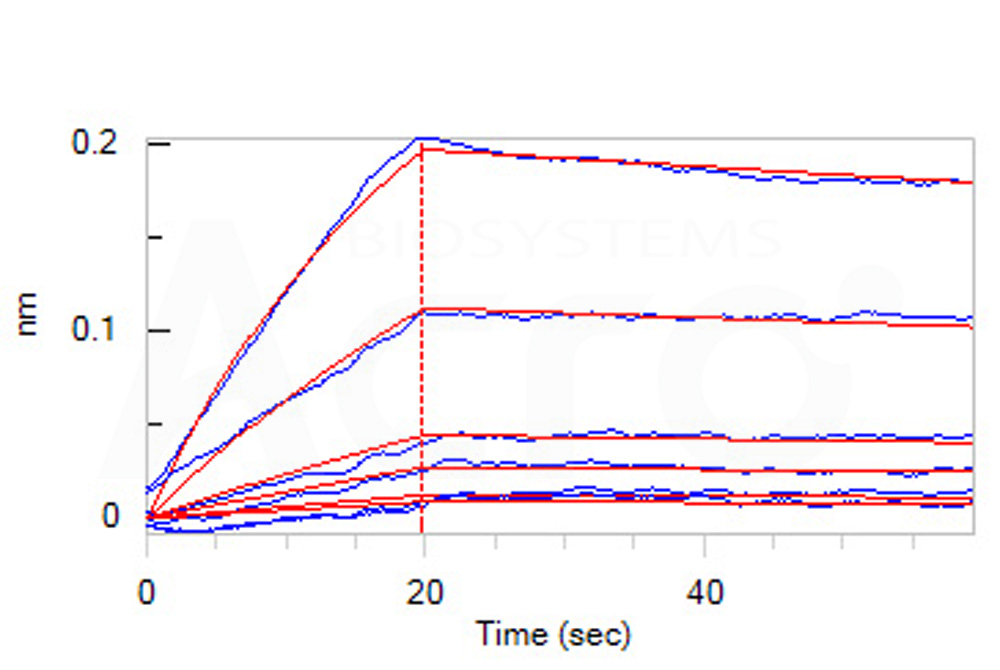
| IL2-H52H8 | Human | Human IL-2 Protein, His Tag | 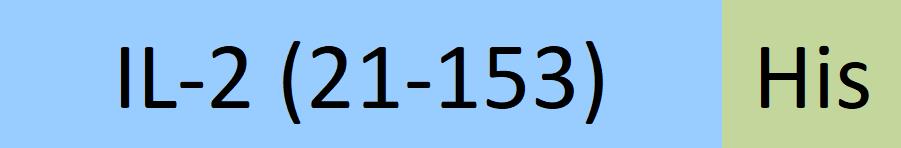 |
|

|
| IL2-H82E4 | Human | Biotinylated Human IL-2 Protein, His,Avitag™ | 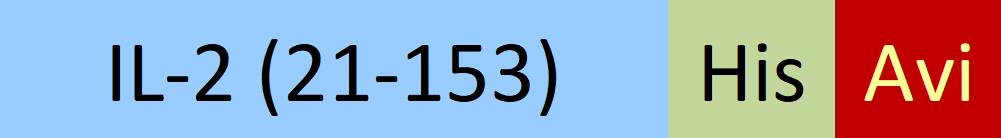 |

|
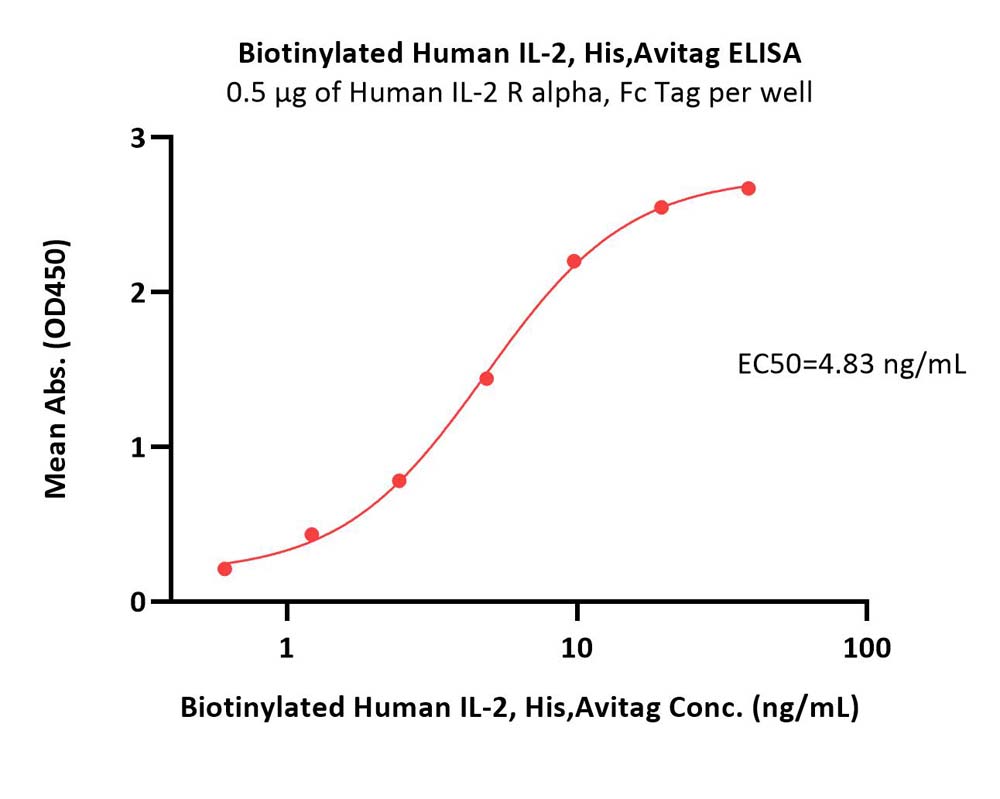

|
| IL2-H82F3 | Human | Biotinylated Human IL-2 Protein, Fc,Avitag™ |  |

|


|

GMP Human IL-2 Protein (Cat. No. GMP-L02H14) stimulates proliferation of CTLL-2 cells. The specific activity of GMP Human IL-2 Protein is ≥ 1.20×10^7 IU/mg, which is calibrated against human Interleukin-2 China National Standard (NIFDC code: 270008) (QC tested). China National Institutes for Food and Drug Control (NIFDC) Standard was prepared and calibrated against human IL-2 WHO International Standard (NIBSC code: 86/500) by NIFDC.
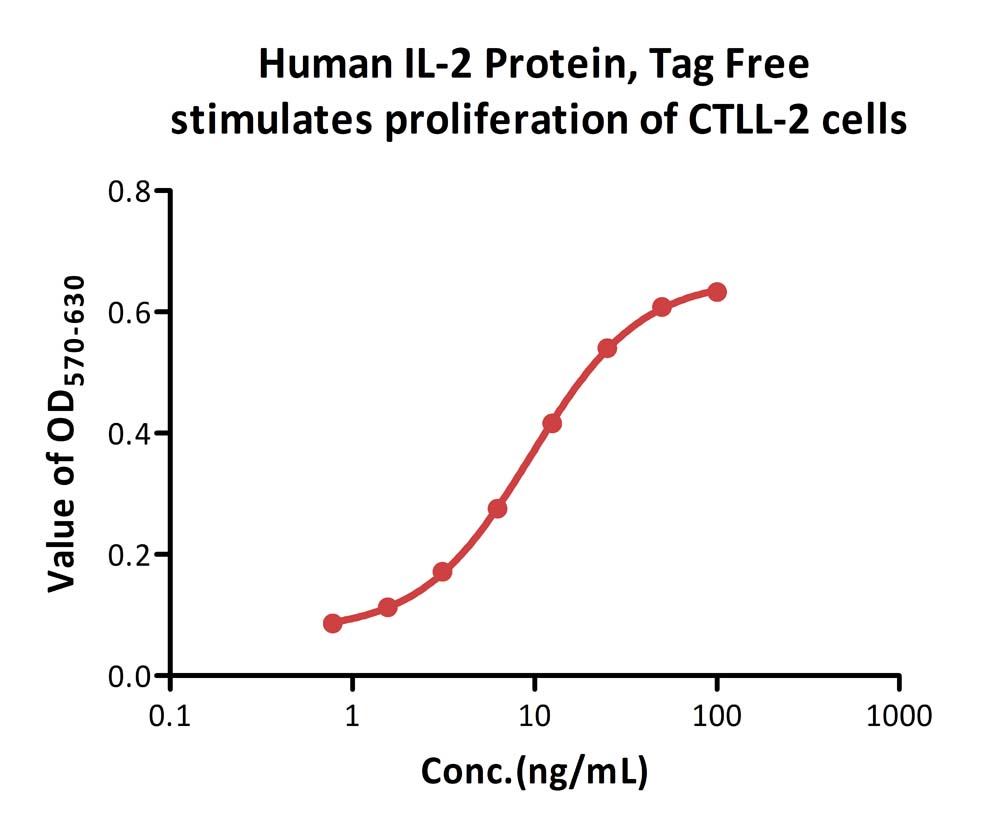
Human IL-2 Protein, Tag Free (Cat. No. IL2-H5215) stimulates proliferation of CTLL-2 cells. The specific activity of Human IL-2 Protein, Tag Free is > 5.00×10^6 IU/mg, which is calibrated against Interleukin-2 (Human, rDNA derived) (2nd International Standard) (NIBSC code: 86/500) (QC tested).

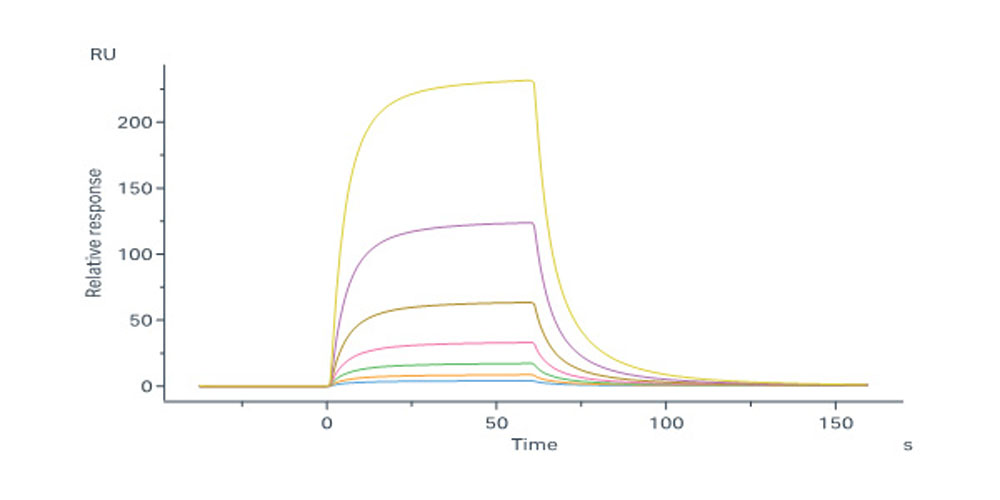
Cynomolgus IL-2 R beta, His Tag (Cat. No. ILB-C52H9) immobilized on CM5 Chip can bind Cynomolgus IL-2, His Tag (Cat. No. IL2-C5249) with an affinity constant of 377 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Japan | Leprosy, Lepromatous | Fujimoto Pharmaceutical | 1982-01-01 | Osteosarcoma; Leprosy, Lepromatous; Drug Resistant Epilepsy; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Waldenstrom Macroglobulinemia; Cholangitis, Sclerosing; HIV Wasting Syndrome; Arachnoiditis; Adenocarcinoma, Clear Cell; Prostatic Neoplasms; Pancreatitis, Chronic; Lymphoma, Follicular; Sarcoma; Xerostomia; Burning Mouth Syndrome; Neoplasm Metastasis; Mycobacterium avium-intracellulare Infection; Vascular Malformations; Amyotrophic Lateral Sclerosis; Melanoma; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Myelodysplastic-Myeloproliferative Diseases; Stomatitis; Erythema Nodosum; Anemia, Sideroblastic; Uterine Neoplasms; Lymphoma, Non-Hodgkin; Glioma; Angiodysplasia; Pelvic Pain; Appendiceal Neoplasms; Lung Neoplasms; Endometrial Neoplasms; Mycobacterium Infections; Gastric Antral Vascular Ectasia; Carcinoid Tumor; Lupus Erythematosus, Discoid; Stomatitis, Aphthous; Rhabdomyosarcoma; | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| T20K | T-20K | Phase 1 Clinical | Cyxone | Multiple Sclerosis | Details |
| RG-6279 | RG-6279 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours | Details |
| S-95007 | S-95007 | Phase 2 Clinical | Laboratoires Servier | Immune System Diseases; Inflammation | Details |
| SAR-444336 | SAR-444336 | Phase 1 Clinical | Sanofi | Inflammation | Details |
| LMBP2-specific TCR-T cell therapy (TCRCure Biopharma) | Phase 2 Clinical | Tcrcure Biopharma Ltd | Nasopharyngeal Carcinoma | Details | |
| SHR-1916 | SHR-1916 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Solid tumours | Details |
| STK-012 | STK-012 | Phase 1 Clinical | Synthekine Inc | Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Microsatellite instability-high cancer; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Darleukin/fibromun | L19-IL-2/ L19-TNF-α | Phase 3 Clinical | Philogen Spa | Carcinoma, Merkel Cell; Carcinoma, Skin Appendage; Basal Cell Nevus Syndrome; Keratoacanthoma; Carcinoma, Squamous Cell; Lymphoma, T-Cell, Cutaneous; Melanoma; Sarcoma, Kaposi | Details |
| ITI-1000 | ITI-1000 | Phase 2 Clinical | Immunomic Therapeutics Inc | Glioblastoma | Details |
| NNC-0361-0041 | NNC0361-0041; NNC-0361-0041 | Phase 1 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 1 | Details |
| XTX-202 | XTX-202 | Phase 2 Clinical | Solid tumours | Details | |
| Interleukin-2 gene therapy (St Jude Children's Research Hospital) | Phase 1 Clinical | St Jude Children's Research Hospital, National Cancer Institute | Neuroblastoma | Details | |
| AU-007 | BD-8; AU-007; BDG-8 | Phase 2 Clinical | Biolojic Design Inc | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| PM-1016 | PM1016; TILT-123 | Phase 2 Clinical | Tilt Biotherapeutics Ltd | Ovarian Neoplasms; Solid tumours; Squamous Cell Carcinoma of Head and Neck; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Hepatocellular; Melanoma | Details |
| EQ101 | EQ101 | Phase 2 Clinical | Equillium Inc | Alopecia; Alopecia Areata | Details |
| Bifikafusp alfa | L19-IL-2 | Phase 3 Clinical | Philogen Spa | Solid tumours; Carcinoma, Basal Cell; Lymphoma, Large B-Cell, Diffuse; Carcinoma, Squamous Cell; Melanoma; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| attIL2-T cell therapy | Phase 1 Clinical | MD Anderson Cancer Center | Soft Tissue Neoplasms; Osteosarcoma; Sarcoma | Details | |
| Saltikva | Phase 2 Clinical | Salspera LLC | Pancreatic Neoplasms | Details | |
| AVB-001 | AVB-001 | Phase 2 Clinical | Avenge Bio Inc | Ovarian Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
This web search service is supported by Google Inc.
