 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| Anti-GCC humanized antibody | Bispecific antibody | Oncology/Cancer | Solid Tumor | Preclinical | Global |
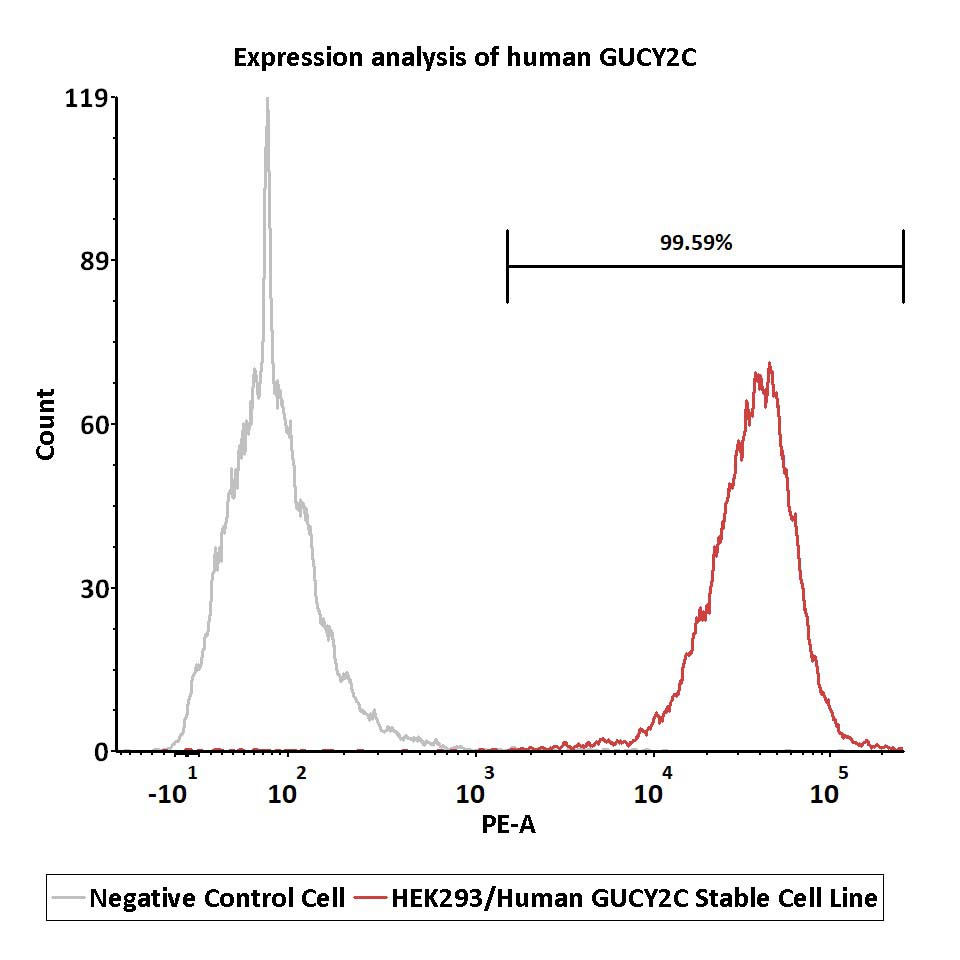
Expression analysis of human GUCY2C on HEK293/Human GUCY2C Stable Cell Line by FACS.
Cell surface staining was performed on HEK293/Human GUCY2C Stable Cell Line or negative control cell using anti-human GUCY2C antibody followed by staining with PE anti-human IgG antibody.
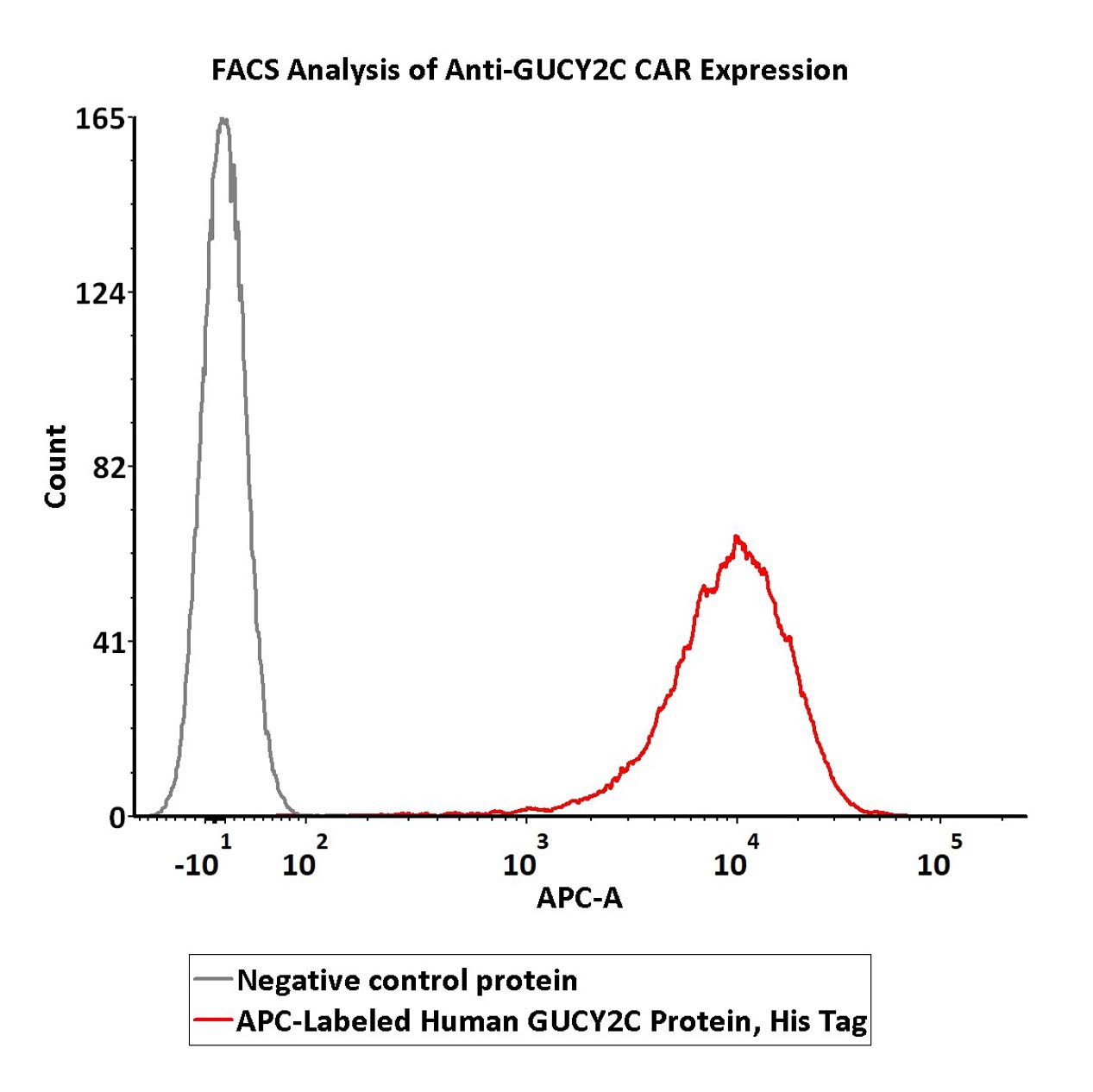
5e5 of anti-GUCY2C CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of APC-Labeled Human GUCY2C Protein, His Tag (Cat. No. GUC-HA2H4) and negative control protein respectively. APC signal was used to evaluate the binding activity (QC tested).

Immobilized Monoclonal Anti-Human GUCY2C Antibody, Human IgG1 at 1 μg/mL (100 μL/well) can bind FITC-Labeled Human GUCY2C, Fc Tag (Cat. No. GUC-HF255) with a linear range of 0.01-0.313 μg/mL (QC tested).

The purity of Mouse GUCY2C Protein, His Tag (Cat. No. GUC-M53H5) is more than 90% and the molecular weight of this protein is around 75-95 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Plecanatide | SP-304; GCRA | Approved | Synergy Pharmaceuticals Inc | Trulance | United States | Constipation; Chronic idiopathic constipation | Salix Pharmaceuticals | 2017-01-19 | Constipation; Irritable Bowel Syndrome; Chronic idiopathic constipation | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| PF-07062119 | PF-07062119 | Pfizer Pharmaceuticals Ltd (China) | Details | ||
| GCC19CART | GCC19CART | Phase 1 Clinical | Innovative Cellular Therapeutics Co Ltd | Rectal Neoplasms; Colorectal Neoplasms | Details |
| IM96 CAR-T cell Therapy | Phase 1 Clinical | Beijing Immunochina Medical Science & Technology Co Ltd | Solid tumours; Neoplasms; Pancreatic Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms | Details |
This web search service is supported by Google Inc.
