 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > Targeting CD20 and CD3, the witness and creator in the process of antibody drug development 
On October 4, 2021, Johnson & Johnson and Xencor reached a cooperation agreement that Johnson & Johnson paid $100 million in advance and made a $25 million equity investment in Xencor. In addition, it needed to pay a milestone amount of $1.188 billion to jointly develop and commercialize plamotamab and novel XmAb® B-cell targeting bispecific antibodies that are designed to conditionally activate T cells through the CD28 co-stimulatory receptor.
In September 2021, the Center for Drug Evaluation (CDE) of China announced that Roche's CD3/CD20 bispecific antibody RO7030816 (Mosunetuzumab) has obtained clinical trial implied permission for the treatment of relapse/ refractory follicular lymphoma.
Targeting CD20 and CD3 can be interestingly considered to have witnessed the entire process in antibody drug development. Early in the mid-nineteenth century, it was found that there were "bactericidin" in the serum of patients infected with certain diseases that could treat related diseases. This was our initial understanding of antibodies. It took nearly a century to propose the model from "bactericidin" to antibody.
When it comes to the development of real therapeutic antibody drugs, it dates back to the 1970s. Milstein and Kohler tried to fuse antibody-producing B cells with tumor cells to develop hybridoma cells that can both secrete antibodies and proliferate indefinitely. This technology is a milestone in the development of therapeutic antibody drugs and Milstein and Kohler also won the Nobel Prize in Physiology or Medicine in 1984.
In the second year of the Nobel Prize for hybridoma technology, the first therapeutic antibody drug Orthoclone OKT3 was approved by the US FDA. This is an antibody that targets CD3 and is used to prevent immune rejection after kidney transplantation. Orthoclone OKT3 is of epoch-making significance as the first approved therapeutic antibody drug. However, due to its sequence is source of mice origin, it is recognized and eliminated by the human immune system as an exogenous protein during the administration process, resulting in a significant decrease in the therapeutic effect after multiple administrations, and may even induce serious immune-related side effects, even death.
The emergence of these adverse events cast a shadow over the development of therapeutic antibody drugs.
On this basis, the murine variable region was chimerized with the human constant region to prepare a chimeric antibody that can recognize the target protein and reduce the proportion of murine sequence, which initially reduces the heterologous rejection caused by sequence. The representative drug among the chimeric antibodies is the Rituxan, the monoclonal antibody targeting CD20 approved by Roche in 1997 for the treatment of B-cell lymphoma. With the continuous progress of antibody drug research and development, subsequent humanized antibodies obtained through sequence modification or optimization. Fully human antibodies obtained through transgenic animals or phage/yeast display technology have gradually matured, making the development of antibody drugs appear an explosive leap.
CD20 (membrane spanning 4-domains A1, MS4A1) is a four-pass transmembrane phosphoprotein, which is involved in regulating the activation and proliferation of B cells. B lymphocytes are differentiated from pluripotent stem cells in the bone marrow. They develop through progenitor B cells, pre-B cells, immature B cells and mature B cells. Mature B cells are released into lymphoid tissues and can continue to differentiate into plasma cells. CD20, is a surface marker of B cell, whose expression initiates somewhat later, and is lost somewhat earlier compared with CD19, during B cell development and differentiation. It is expressed from pre-B cells to mature B cells, but not on pro- B cells and plasmablasts.
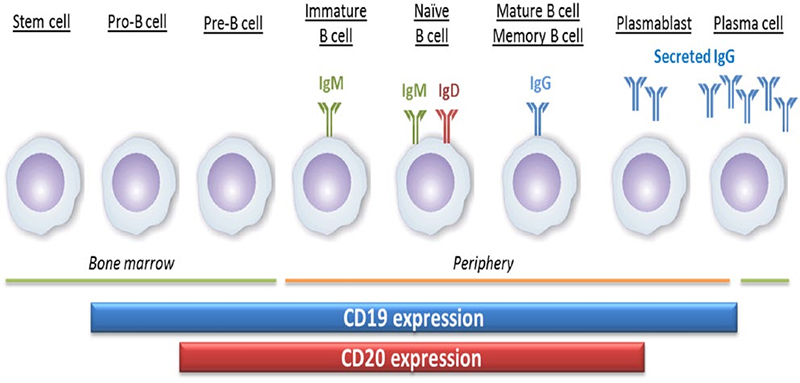
The expression of CD20 and CD19 during the development of B cells
The expression of CD20 on B cell-derived lymphoma, leukemia and other tumor cells, and B cells involved in inflammatory and immune-related diseases makes it a potential therapeutic target for lymphoma, leukemia and autoimmune diseases.
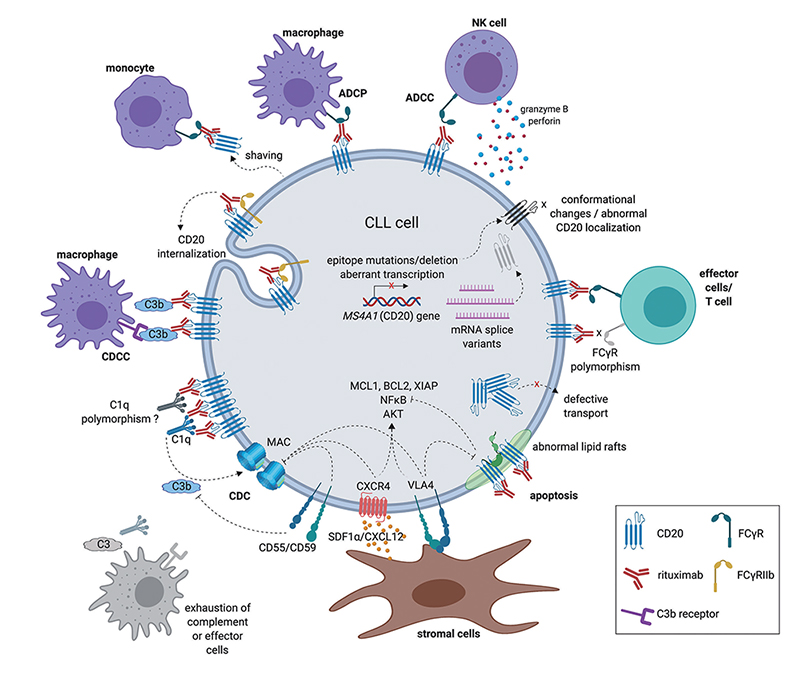
The mechanism of action of anti-CD20 antibodies
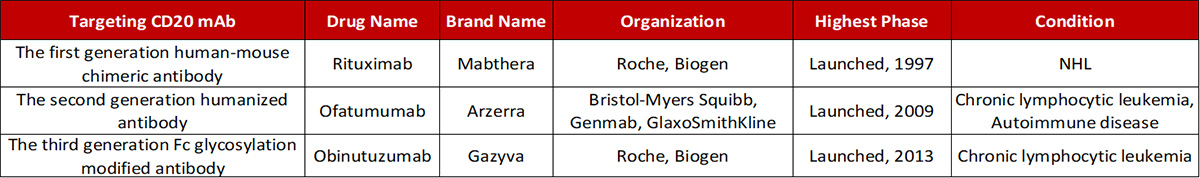
Antibody drugs targeting CD20 have undergone three generations of innovation: the first generation is Rituximab, a chimeric IgG1 mAb that binds to the B lymphocyte surface antigen CD20. It is generally thought that Rituximab depletes B lymphocytes via ADCC and complement-dependent cytotoxicity (CDC) mechanisms. The second generation is Ofatumumab, a fully humanized IgG1 anti-CD20 mAb. Although the immunogenicity and side effect are reduced, the antibody specificity and the affinity of the antigen binding have a certain decline. The third generation is Obinutuzumab, which Fc region was glycosylated to improve binding capacity to effector cells, including neutrophils, natural killer cells, thereby possibly enhancing ADCC. However, clinical data shows that although the anti-CD20 monoclonal antibody has developed to the third generation, the leading position of Rituximab has not been shaken.
Progress in the development of mAbs targeting CD20
Not limited to the field of monoclonal antibody drugs, CD20 can recruit T cells to B cells by binding to CD3 and other targets for the development of bispecific antibodies, which in turn achieve the killing effect of T cells on B cells.
Currently in the field of CD20/CD3 bispecific antibody development, Epcoritamab (GEN3013) developed by Genmab is a subcutaneously injected bispecific antibody and is currently being studied for the treatment of B-cell non-Hodgkin's lymphoma. Roche has developed two bispecific antibodies with different structural forms that simultaneously target CD20/CD3: Mosunetuzumab and Glofitamab. At ASH annual conference in 2020, Roche disclosed the results of the drug's clinical trials: the overall effective rate reached 86% in patients who received Mosunetuzumab combined with CHOP in the treatment of relapsed/refractory non-Hodgkin's lymphoma, of which 71% patients achieved complete remission. The current clinical studies of Mosunetuzumab cover multiple indications such as diffuse large B-cell lymphoma, non-Hodgkin's lymphoma, and follicular lymphoma.
Glofitamab is a bispecific antibody with a special structure developed by Roche, which contains two domains that can bind to CD20 as well as one that binds to CD3.
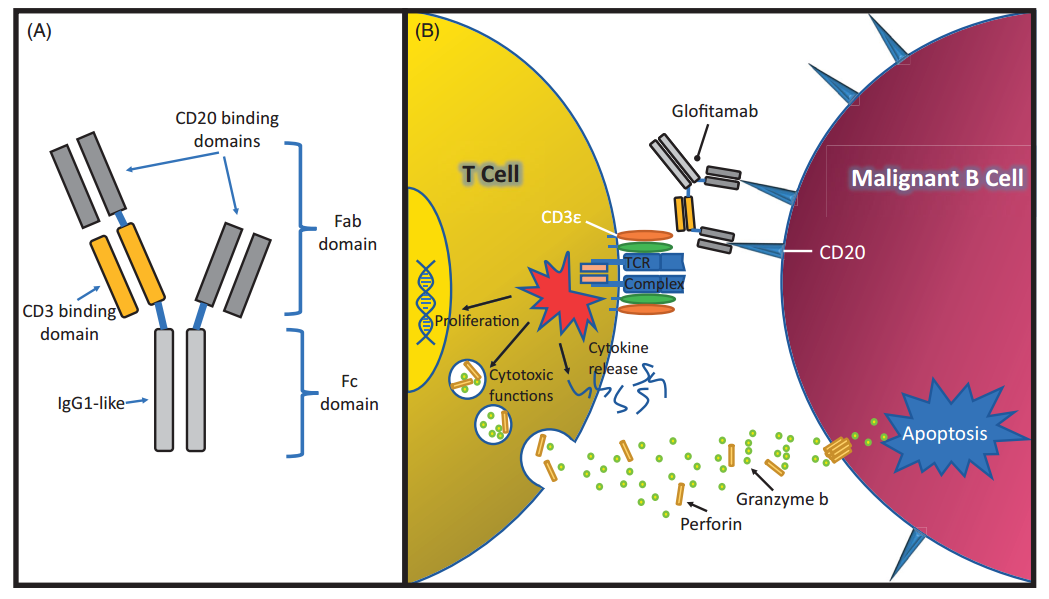
The structure and mechanism of Glofitamab
At ASH annual conference in 2019, Roche announced the clinical trial progress data of Glofitamab combined with the third-generation CD20 mAb (Otuzumab) in the treatment of relapsed/refractory non-Hodgkin's lymphoma: the objective response rate (ORR) of combined treatment was 54% (n=15/28), and the complete response rate (CR) was 46% (n=13/28).
The huge market brought by the CD20 monoclonal antibody has caused global pharmaceutical companies to join the track. In order to avoid excessive competition and achieve product differentiation, pharmaceutical companies have turned to the research and development of bispecific antibodies, which has set off a new upsurge in CD20/CD3 bispecific antibody drug development.
Full-length CD20 and CD3 series target proteins are available from ACROBiosystems To facilitate the development of drugs targeting CD20, ACROBiosystems has specially set up multiple technology platforms to develop full-length four-pass transmembrane target protein CD20 expressed by HEK293.
Full-length CD20 proteins
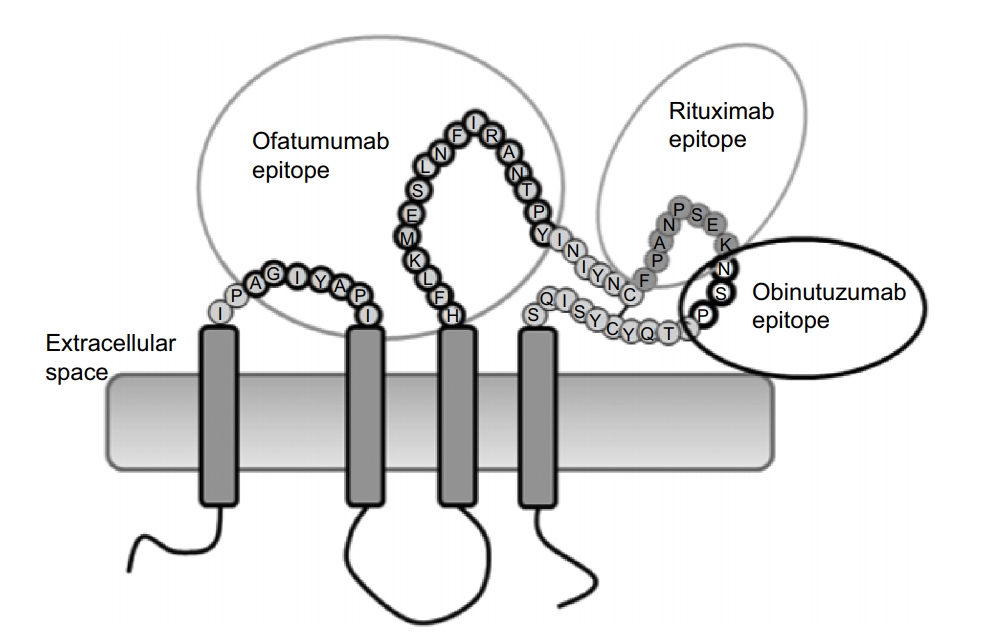
 Native conformation and complete epitope: the recognition epitope of Rituximab and Obinutuzumab is a large loop of the extracellular domain of CD20, and Ofatumumab binds to both large loop and small loop
Native conformation and complete epitope: the recognition epitope of Rituximab and Obinutuzumab is a large loop of the extracellular domain of CD20, and Ofatumumab binds to both large loop and small loop
 Larger product catalog: CD20-Nanodisc, CD20-DDM/CHS, CD20-VLP, including unconjugated and biotinylated protein
Larger product catalog: CD20-Nanodisc, CD20-DDM/CHS, CD20-VLP, including unconjugated and biotinylated protein
 High bioactivity: the biological activity is verified by binding Rituximab and Ofatumumab, showing high affinity at the nanomolar level, all protocols offered for free
High bioactivity: the biological activity is verified by binding Rituximab and Ofatumumab, showing high affinity at the nanomolar level, all protocols offered for free
 Suitable for ELISA/SPR/BLI/ ELISA/ SPR/ BLI/ FACS
Suitable for ELISA/SPR/BLI/ ELISA/ SPR/ BLI/ FACS
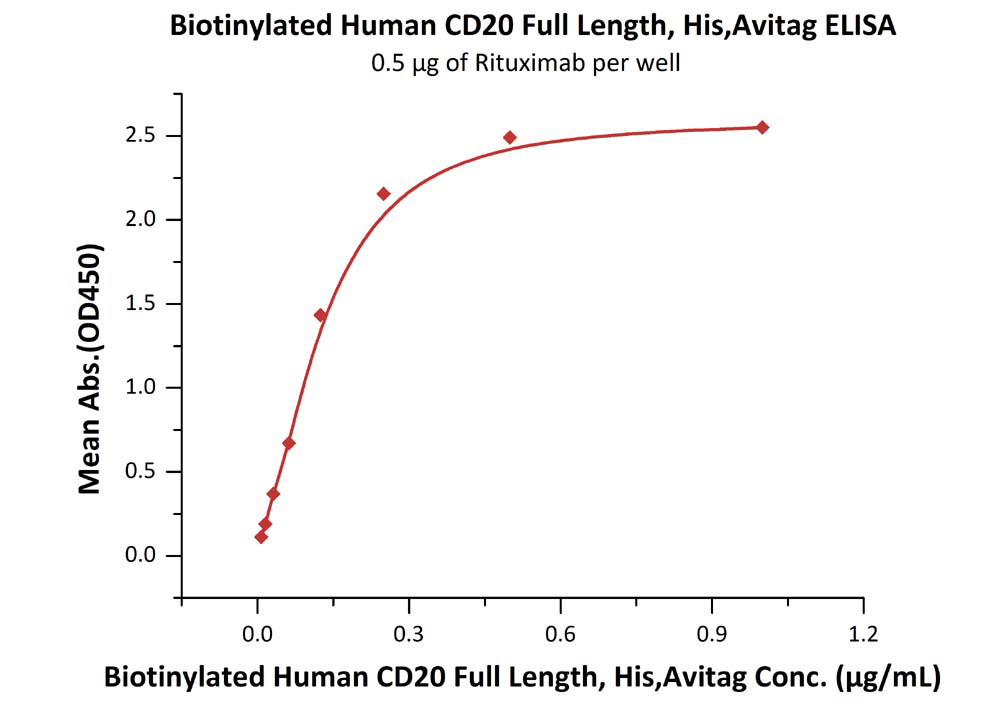
Immobilized RTX at 5 μg/mL (100 μL/well) can bind Biotinylated Human CD20 Full Length, His, Avitag (Cat. No. CD0-H82E3) with a linear range of 0.008-0.125 μg/mL.
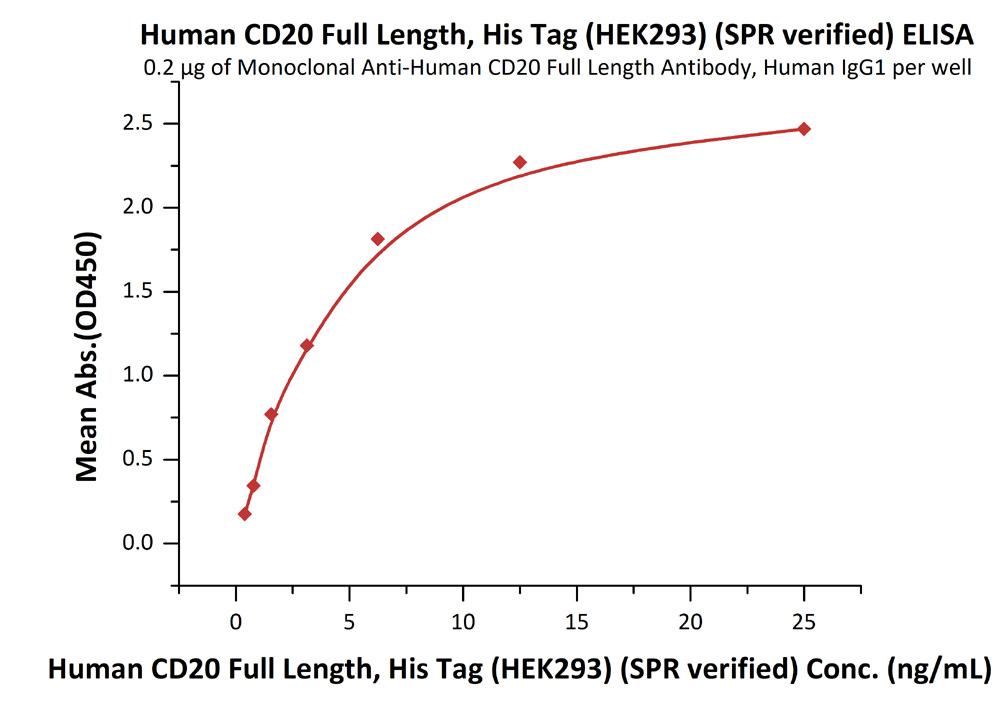
Immobilized anti-CD20 antibody at 2 μg/mL (100 μL/well) can bind Human CD20 Full Length, His Tag, HEK293 (SPR verified) (Cat. No. CD0-H52H3) with a linear range of 0.4-6 ng/mL (in presence of DDM and CHS).
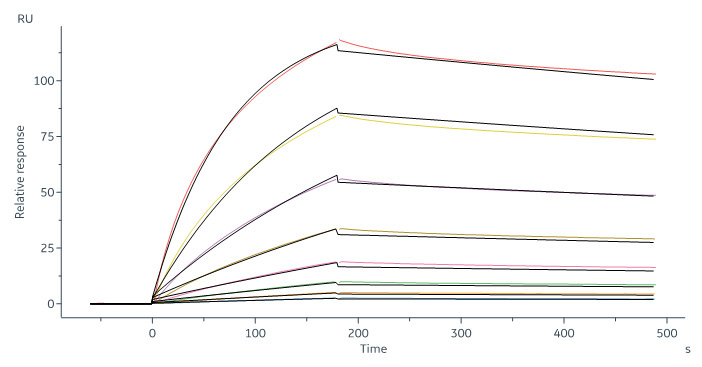
Human CD20 Full Length, His Tag, HEK293 (SPR verified) (Cat. No. CD0-H52H3) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind RTX with an affinity constant of 6.21 nM as determined in a SPR assay (in presence of DDM and CHS) (Biacore 8K).
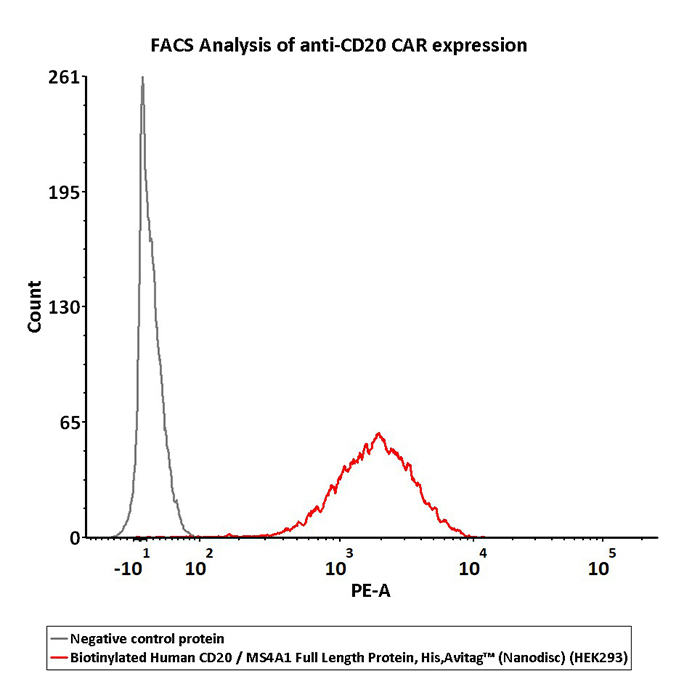
2e5 of CD20-CAR-293 cells transfected with anti-CD20-scFv were stained with 100 μL of 3 μg/mL of Biotinylated Human CD20 Full Length, His, Avitag (Cat. No. CD0-H82E3) and negative control protein respectively, washed and then followed by PE-SA and analyzed with FACS (QC tested).
>>> Click to learn more about full-length CD20 proteins and verification data with free protocols
To accelerate the research and development of bispecific antibody drugs, ACROBiosystems provides a series of high-quality CD3 proteins.
 CD3 proteins
CD3 proteins
 Larger product catalog
Larger product catalog
5 molecules: CD3ε, CD3δ, CD3γ, CD3E&CD3D (CD3ε& CD3δ), CD3E&CD3G (CD3ε& CD3γ)
Various tags: his, his&Avi, Fc, Llama Fc, Flag
Various species: Human, Mouse, Rat, Rabbit, Cynomolgus
 Homogeneous structure
Homogeneous structure
CD3E&CD3D and CD3E&CD3G have been confirmed as 1:1 heterodimer respectively verified by non-reductive electrophoresis and MALS.
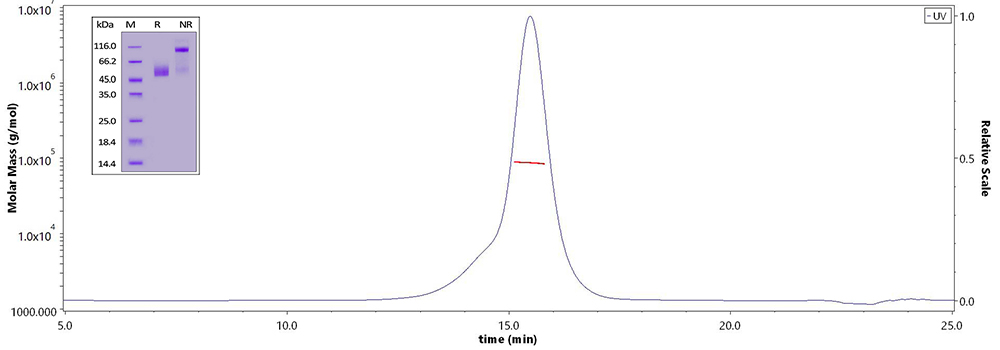
Human CD3E&CD3D Heterodimer Protein (Cat. No. CDD-H52Wa) on SDS-PAGE under reducing (R) and non-reducing(NR)condition and the purity of the protein is greater than 95%. The purity of the protein is more than 85% and around 80-90 kDa verified by SEC-MALS.
 High batch-to-batch consistency
High batch-to-batch consistency
CD3 proteins are under strict quality control to guarantee the minimal batch-to-batch differences.
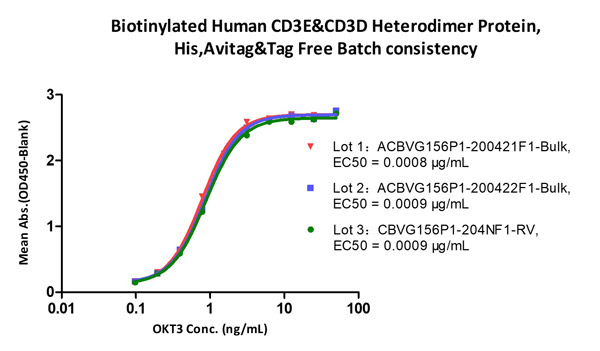
Immobilized Biotinylated Human CD3E&CD3D Heterodimer Protein, His, Avitag&Tag Free (Cat. No. CDD-H82W6) at 1 μg/mL (100 μL/well) on Streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate, can bind Monoclonal Anti-Human CD3 Antibody, Mouse IgG2a (Cat. No. CDE-M120a) with a linear range of 0.2-6 ng/mL (QC tested), and batch differences EC50<0.0001 μg/mL.
 High bioactivity, in line with drug development requirements
High bioactivity, in line with drug development requirements
High bioactivity of CD3 proteins is verified by binding with common antibodies such as OKT3, SP34-2, UCHT1 and BCMA×CD3.
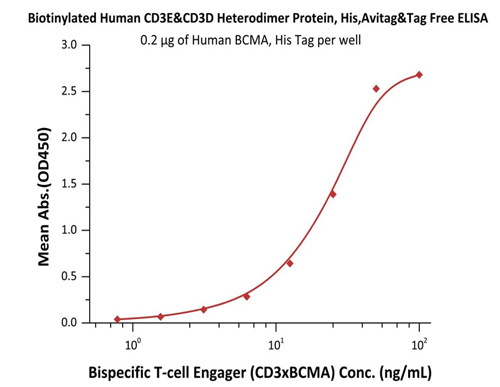
Immobilized Human BCMA, His Tag (Cat. No. BCA-H522y) at 2 μg/mL, add increasing concentrations of Bispecific T cell Engager (CD3 X BCMA) in 10% human serum and then add Biotinylated Human CD3E&CD3D Heterodimer Protein, His, Avitag&Tag Free (Cat. No. CDD-H82W6) at 0.2 μg/mL. Detection was performed using HRP-conjugated streptavidin with sensitivity of 15 ng/mL.
>>>Click to learn more about CD3 proteins and verification data with free protocols
>>>Find out more bispecific antibody targets
1. Forsthuber TG, Cimbora DM, Ratchford JN, Katz E, Stüve O. B cell-based therapies in CNS autoimmunity: differentiating CD19 and CD20 as therapeutic targets. Ther Adv Neurol Disord. 2018 Mar 21;11:1756286418761697. doi: 10.1177/1756286418761697. PMID: 29593838; PMCID: PMC5865455.
2. Pavlasova G, Mraz M. The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy. Haematologica. 2020 Jun;105(6):1494-1506. doi: 10.3324/haematol.2019.243543. PMID: 32482755; PMCID: PMC7271567.
3. Adrian Minson, Michael Dickinson. Glofitamab CD20-TCB bispecific antibody. Leukemia & Lymphoma, DOI: 10.1080/10428194.2021.1953016
4. Hill BT, Kalaycio M. Profile of obinutuzumab for the treatment of patients with previously untreated chronic lymphocytic leukemia. Onco Targets Ther. 2015 Aug 31;8:2391-7. doi: 10.2147/OTT.S68770. PMID: 26366093; PMCID: PMC4562745.
This web search service is supported by Google Inc.
