 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!

5e5 of anti-Mucin-1 (F1C) CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of APC-Labeled Human Mucin-1 Protein, His Tag (Cat. No. MU1-HA2H8) and negative control protein respectively. APC signal was used to evaluate the binding activity (QC tested).
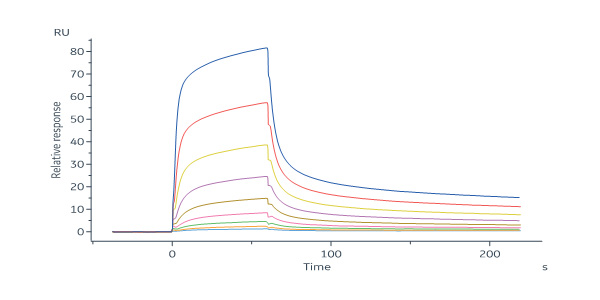
Mouse Mucin-1 (21-535) Protein, His Tag (Cat. No. MU1-M52H4) immobilized on CM5 Chip can bind anti-mMUC1-mAb with an affinity constant of 302 nM as determined in a SPR assay (Biacore 8K) (QC tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Anti-MUC1 CAR T cell (Guangzhou Anjie Biomedical) | Phase 2 Clinical | Guangzhou Anjie Biomedical Technology | Esophageal Neoplasms; Prostatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Recombinant HSP-MUC1 fusion protein (Newsummit) | Phase 1 Clinical | Shanghai Xinshengyuan Biological Medicine Co Ltd | Breast Neoplasms | Details | |
| Anti-MUC1 CAR T-cell therapy (Guangzhou Anjie Biomedical Technology/University of Technology Sydney) | Phase 2 Clinical | The First Affiliated Hospital Of Guangdong Pharmaceutical University, Guangzhou Anjie Biomedical Technology, University of Sydney | Carcinoma, Non-Small-Cell Lung | Details | |
| Anti MUC 1 chimeric antigen receptor T cell therapy (Innovative Cellular) | Phase 1 Clinical | Innovative Cellular Therapeutics Co Ltd | Pancreatic Neoplasms; Breast Neoplasms; Lung Neoplasms | Details | |
| ICTCAR-046 | ICTCAR-046 | Clinical | Innovative Cellular Therapeutics Co Ltd | Pancreatic Neoplasms | Details |
| BromAc | Mucpharm Pty Ltd | Details | |||
| Cantuzumab ravtansine | Immunogen Inc | Details | |||
| Anti-MUC1 CAR-T cell therapy (PersonGen) | Persongen Biotherapeutics (Suzhou) Co Ltd | Details | |||
| huMNC2-CAR44 | huMNC2-CAR44 | Minerva Biotechnologies | Details | ||
| CART-TnMUC1 | Phase 1 Clinical | University Of Minnesota, University Of Pennsylvania | Ovarian Neoplasms; Triple Negative Breast Neoplasms; Multiple Myeloma; Carcinoma, Pancreatic Ductal; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| DXC-005 | DXC-005 | Phase 1 Clinical | Hangzhou Dac Biotech Company Ltd | Solid tumours | Details |
| P-MUC1C-ALLO1 | P-MUC1C-ALLO1; P-MUC1C-101 | Phase 1 Clinical | Poseida Therapeutics Inc | Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Pancreatic Neoplasms; Nasopharyngeal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Anti-MUC1 monoclonal antibody (OncoQuest) | AR20.5; mAb-AR20.5; Anti-MUC1 AR20.5 | Phase 2 Clinical | Altarex | Pancreatic Neoplasms | Details |
| GO-2032c | GO-203-2C | Phase 2 Clinical | Genus Oncology Llc, Dana-Farber Cancer Institute | Solid tumours; Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| M-1231 | M-1231 | Phase 1 Clinical | Emd Serono Research & Development Institute Inc, Merck Serono | Solid tumours; Esophageal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| MUC1-Poly-ICLC | MUC1-poly-ICLC | Phase 2 Clinical | University Of Pittsburgh | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Adenoviral MUC1 vaccine (Etubics) | ETBX-061; Ad5-MUC1 | Phase 2 Clinical | Etubics Corp, Immunitybio Inc, Nantkwest Inc | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Squamous Cell; Lymphoma, Non-Hodgkin; Lung Neoplasms; Colorectal Neoplasms; Prostatic Neoplasms; Ovarian Neoplasms; Chordoma; Carcinoma, Transitional Cell; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Neoplasms; Colonic Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Merkel Cell | Details |
| Cancer vaccine (BN ImmunoTherapeutics/National Cancer Institute) | CVAC-301; CV-301 | Phase 2 Clinical | Therion Biologics | Solid tumours; Ovarian Neoplasms; Intestinal Neoplasms; Cystadenocarcinoma; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Melanoma; Adenocarcinoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| MUC1-peptide-DC-CTL (Beijing Doing Biomedical) | Phase 1 Clinical | Stomach Neoplasms | Details | ||
| MUC1-gene-DC-CTL (Beijing Doing Biomedical) | Phase 1 Clinical | Stomach Neoplasms | Details |
This web search service is supported by Google Inc.
