 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| SCCHO-ATP171 | Human | CHO/Human CD79B Stable Cell Line Development Service | |||
| CDB-HP2H6 | Human | PE-Labeled Human CD79B Protein, His Tag (Site-specific conjugation) |  |
||
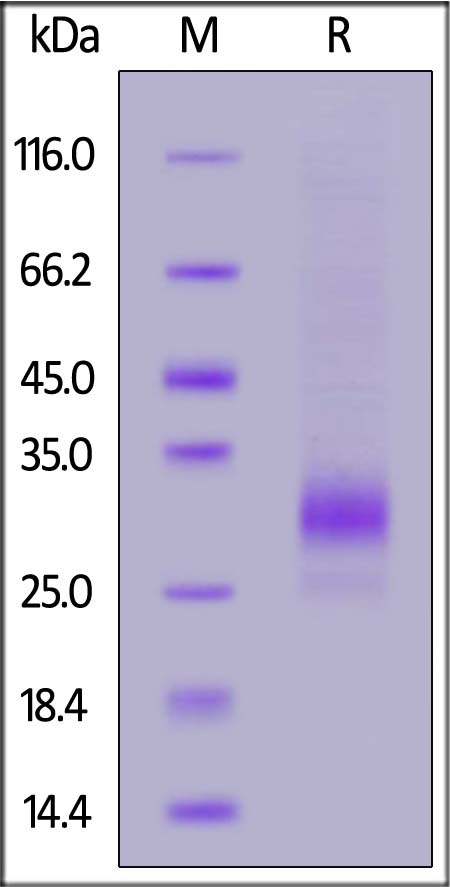
| CDB-C82E5 | Cynomolgus | Biotinylated Cynomolgus CD79B Protein, His,Avitag™ |  |
|
|
| CDB-C52H3 | Cynomolgus | Cynomolgus CD79B Protein, His Tag (MALS verified) |  |

|
|
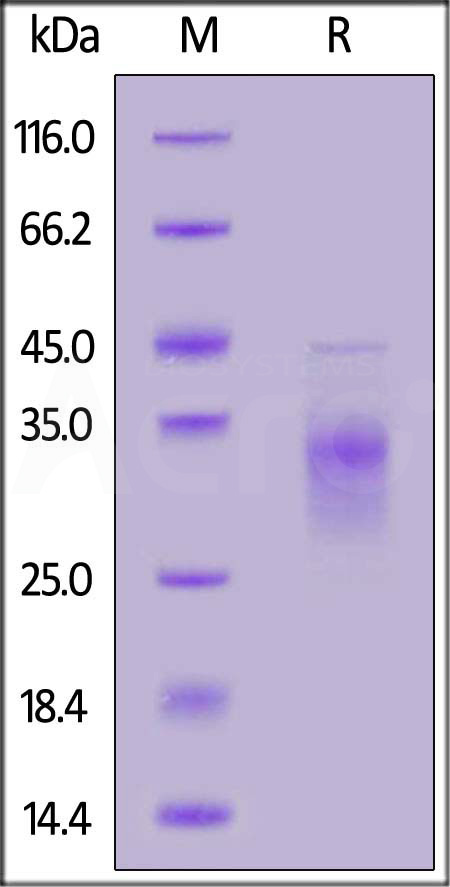
| CDB-H52H3 | Human | Human CD79B Protein, His Tag (MALS verified) |  |
|
|
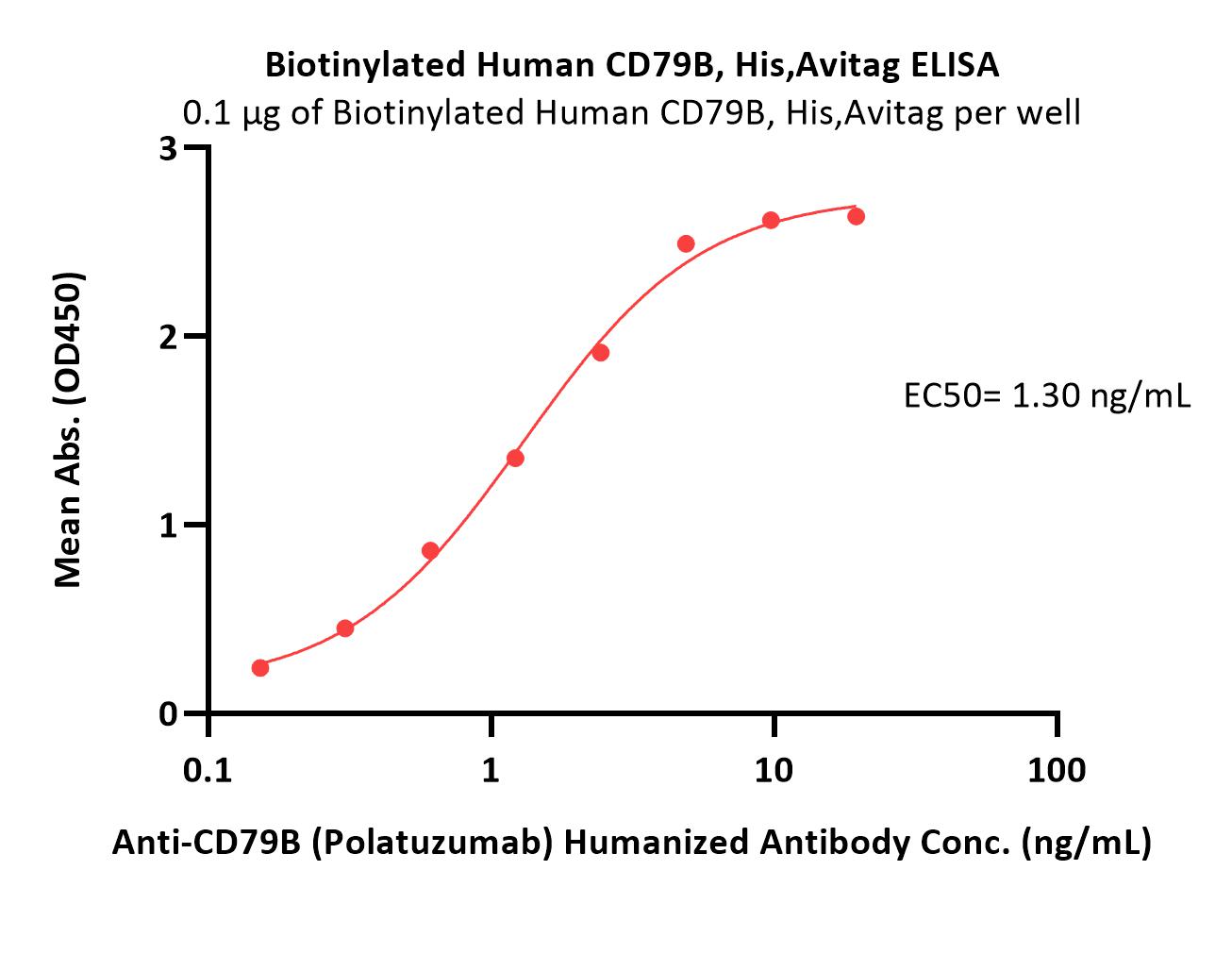
| CDB-H82E3 | Human | Biotinylated Human CD79B Protein, His,Avitag™ (MALS verified) |  |
|
|
| CDB-M52H3 | Mouse | Mouse CD79B Protein, His Tag (MALS verified) |  |

|
|
| CDB-M5253 | Mouse | Mouse CD79B Protein, Fc Tag (MALS verified) |  |

|
|
| CDB-M52H4 | Mouse | Mouse CD79B Protein, His Tag, low endotoxin (MALS verified) |  |
|
|
| CDB-H5259 | Human | Human CD79B Protein, Fc Tag |  |

|
|
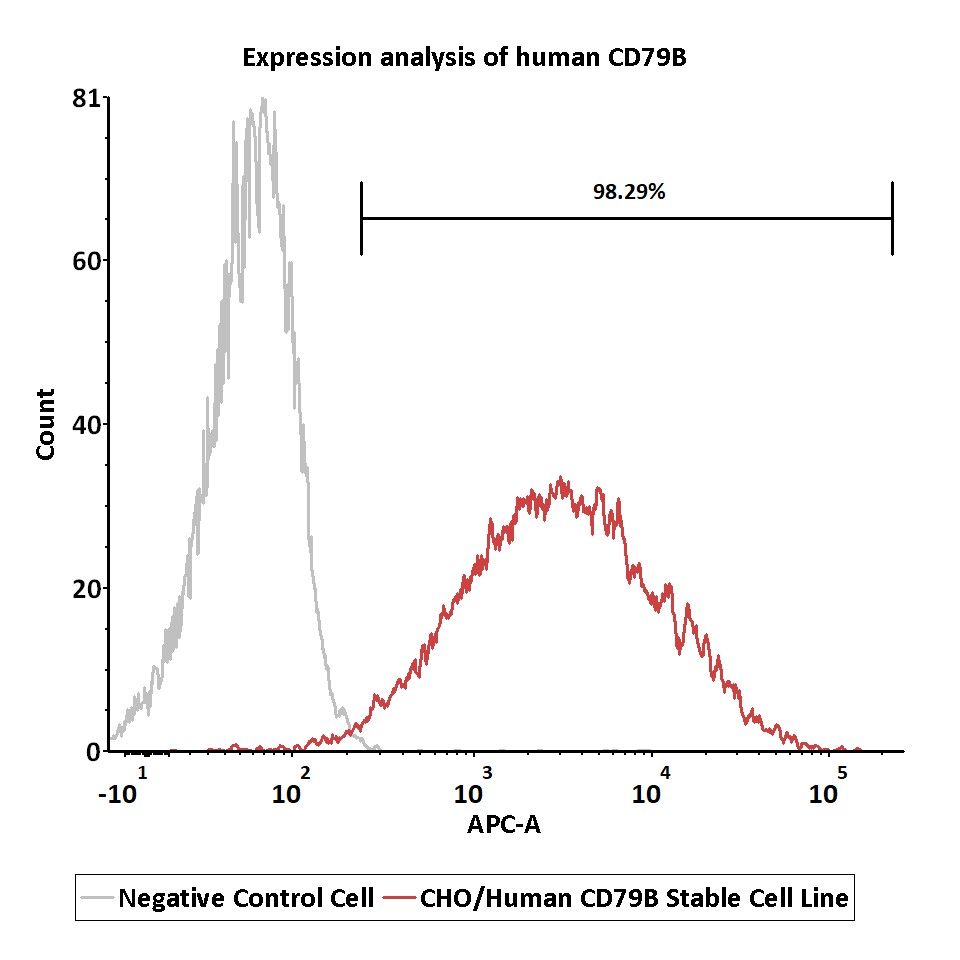
Expression analysis of human CD79B on CHO/Human CD79B Stable Cell Line by FACS.
Cell surface staining was performed on CHO/Human CD79B Stable Cell Line or negative control cell using APC-labeled anti-human CD79B antibody.

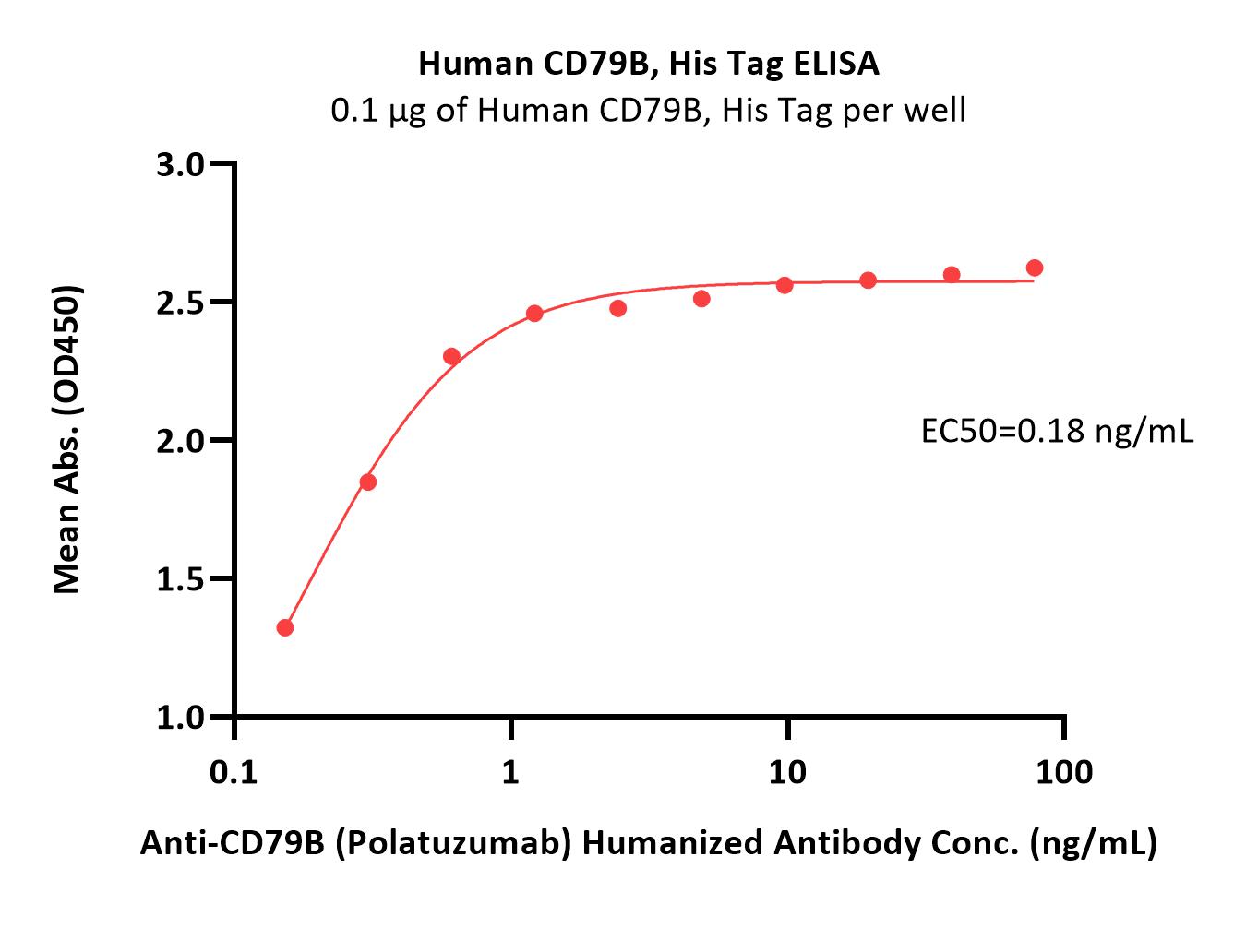
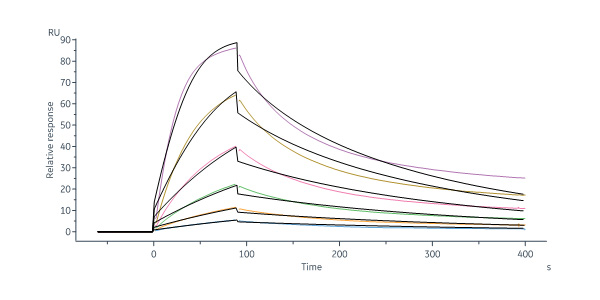
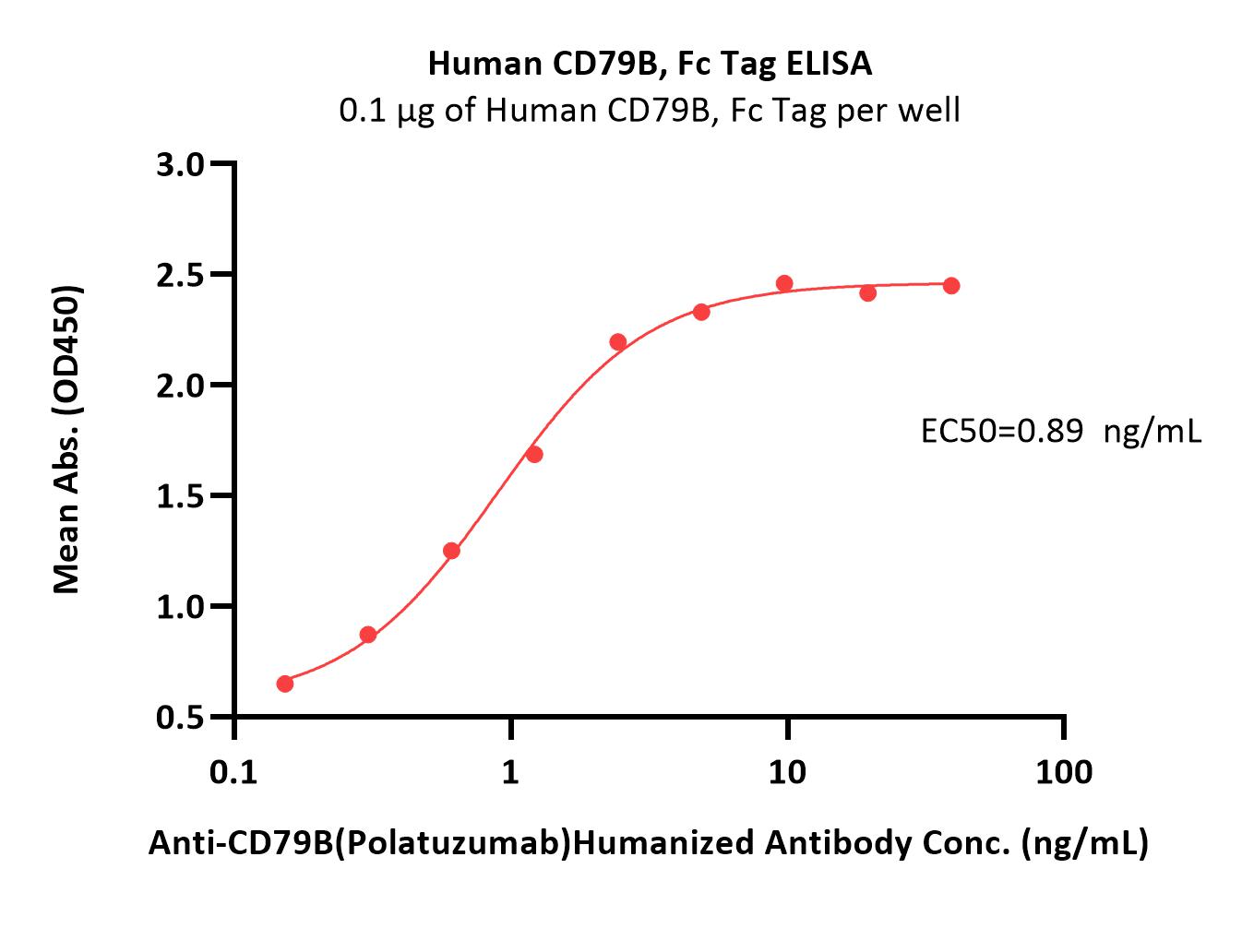
Polatuzumab captured on Protein G Chip can bind Human CD79B, His Tag (Cat. No. CDB-H52H3) with an affinity constant of 1.97 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Polatuzumab vedotin | RG-7596; RO-5541077-000; FCU-2711; DCDS-4501A; RO-5541077 | Approved | Seattle Genetics Inc, Genentech Inc | Polivy | Japan | Lymphoma, Large B-Cell, Diffuse | F. Hoffmann-La Roche Ag | 2019-06-10 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| NBT-508 | Phase 1 Clinical | Newbio Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| Iladatuzumab vedotin | DCDS-0780A; RO-7032005 | F. Hoffmann-La Roche Ltd | Details | ||
| TolDCB29 | Phase 2 Clinical | Umc Utrecht | Arthritis, Rheumatoid | Details | |
| CD19/79b Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR CD19/79b | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell | Details |
| PRV-3279 | MGD-010; PRV-3279; CD32BxCD79B | Phase 2 Clinical | Macrogenics Inc | Drug-Related Side Effects and Adverse Reactions | Details |
This web search service is supported by Google Inc.
