Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > Omicron Antigens and Antibodies at Gram-level Supply: Backing Up Diagnostic Reagents Development To aid the global fight against the Omicron wave of COVID-19 pandemic, ACROBiosystems established an emergency response team to address the lab and industrial need for viral mutant proteins. In just 10 days, a collection of high-quality Omicron antigens, antibodies and other reagents are now in stock at gram-level. To secure your products, please do not hesitate to contact us with any questions or special needs.
Timeline of Omicron development
- On Nov 24th, South Africa reported to the World Health Organization (WHO) the variant B.1.1.529 for the first time, which was found related to the soaring cases in the country that month.
- On Nov 26th, WHO held an emergency meeting to discuss the impact of B.1.1.529, and swiftly designated it Omicron, the fifth variant of concern (VOC).
- On Dec 8th, WHO announced the variant had been detected in 57 countries.
- On Dec 13th, the first death of a person with Omicron was reported in the UK.
Diagnosis of Omicron
In response to the continued emergence of new variants of SARS-CoV-2, the Food and Drug Administration (FDA) has revised the EUAs of certain molecular, antigen, and serology tests to establish additional Conditions of Authorization on Sep 23rd, 2021. The revision requires test developers to update their authorized labeling and evaluate the impact of SARS-CoV-2 viral mutations on their test's performance. If potential impacts are identified, the EUA holder must communicate with the FDA and end users about the potential risk that the presence of the mutations may have on test performance. Due to the astonishing number of mutations, the impact of Omicron on current diagnostic reagents are highly concerned.
Deletion of amino acids HV69-70 within the spike gene of Omicron can result in an undetectable S-gene target (S-gene target failure, SGTF) for some real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing methods, which makes it highly predictive of Omicron. With the emergence of Omicron, a rapid increase in the proportion of SARS-CoV-2 samples with SGTF has been identified and the data is collected for analysis of Omicron’s estimated growth rate. However, latest reports have found a few sub-lineages of Omicron that evolved to miss the SGTF marker, which might compromise the detection efficiency by conventional PCR methods.
Antigen tests is an important complement to molecular tests in screening of SARS-CoV-2 variants. Antigen detection methods that can efficiently detect Omicron mutant strains will play a critical role to meet the global need for detection, prevention and control of Omicron.
With the increasing coverage of immunization either through natural infection or vaccination, the significance of serological antibody tests has also become prominent. Tests that detect antibodies (for example, IgM, IgG) to the SARS-CoV-2 virus is a direct and reliable way to evaluate the degree of herd immunity in a population.
As the world's leading supplier of protein reagents for COVID-19 detection, ACRO is committed to supporting the development of diagnostic tools with high sensitivity and specificity since the outbreak of COVID-19. Our quality and effort have been recognized by many of our IVD partners.
For the development of Omicron diagnostic reagents, ACROBiosystems can now provide:
Spike trimer/RBD/S1/N protein with guaranteed high purity and bioactivity;
High-sensitivity nucleocapsid antibody pair (detect Omicron-N at 0.39 ng/mL sensitivity)
All products are now available in gram-level supply!
ACROBiosystems has now developed all necessary antigen proteins required for the development of Omicron detection tests, including full-length spike trimer, S RBD, NTD, S1, and Nucleocapsid protein.
HEK293 eukaryotic expression system
High protein purity verified by MALS (>95%)
Binding bioactivity verified by ELISA/SPR/BLI
Customized products available
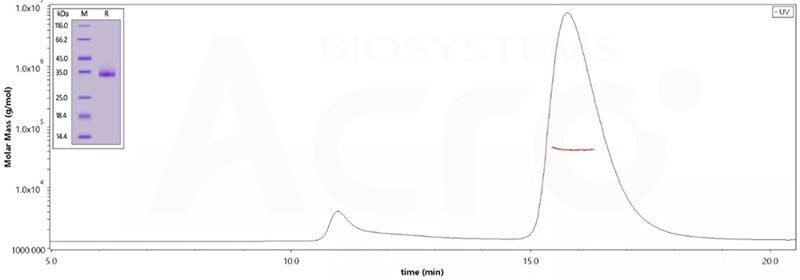
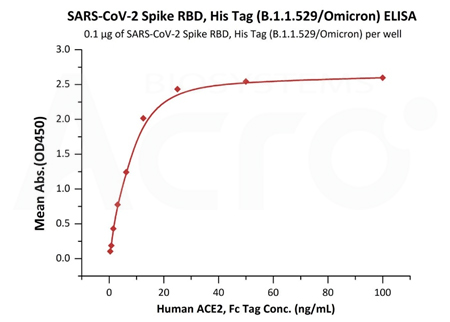
The purity of (Cat.No. SPN-C52Hz) is 95% as determined by SDS-PAGE and 96.8% as determined by SEC-MALS
As the world's leading supplier of protein reagents for COVID-19 diagnostic tests, ACRO is committed to developing high-quality products and technologies to address the urgent needs of the IVD industry. We guarantee consistent product quality, strong technical services and stable global supply for all customers and partners.
This web search service is supported by Google Inc.