 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
To support the research of new VOC variant Omicron of SARS-CoV-2, ACROBiosystems immediately launched an emergency response team to develop related research tools, including antigens, antibodies, pseudoviruses and ELISA kits. We have mobilized all forces to accelerate evaluation of this new emerging variant and the development of vaccines, neutralizing antibodies, antiviral drugs and detection reagents that target Omicron.
At present, ACROBiosystems has successfully produced high-quality full-length Spike trimer and RBD antigen proteins of Omicron mutant that cover all 35 spike mutation sites. Furthermore, we have also successfully screened neutralizing antibodies from our antibody library, which can block Omicron spike-ACE2 interaction.
Other Omicron related products, including Omicron Spike S1, NTD, N protein antigens and Omicron pseudovirus, will be available to fulfill worldwide supply within this week to support global scientific research and industrial production.
High purity: the purity of full-length Spike trimer is 96.8% as verified by SEC-MALS
A complete set of reagents available: Omicron antigens, Nabs, pseufoviruses, ELISA kits, etc.
SARS-CoV-2 Spike Trimer, His Tag (B.1.1.529/Omicron) (Cat.No. SPN-C52Hz)
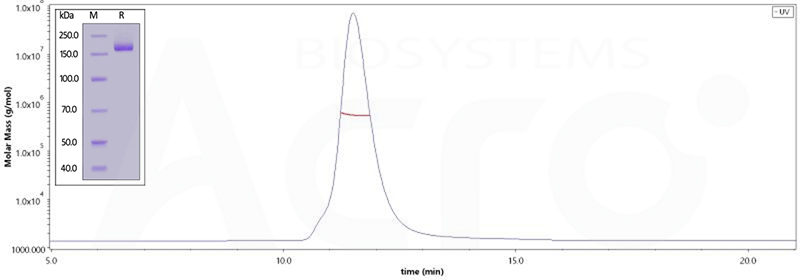
The purity of (Cat.No. SPN-C52Hz) is 95% as determined by SDS-PAGE and 96.8% as determined by SEC-MALS
SARS-CoV-2 Spike RBD,His Tag (B.1.1.529/Omicron) (Cat.No.SPD-C522e)
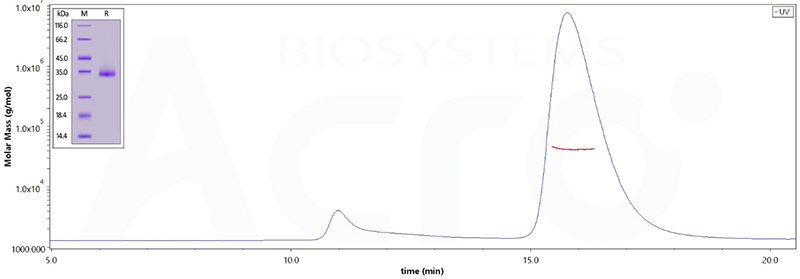
The purity of (Cat.No. SPD-C522e) is 95% as determined by SDS-PAGE and 90.5% as determined by SEC-MALS
Anti-SARS-CoV-2 Spike RBD Antibody, Chimeric mAb, Human IgG1 (AM130) (Cat.No. S1N-M130 )
Anti-SARS-CoV-2 Spike RBD Broadly Neutralizing Antibody, Human IgG1 (Cat.No.SPD-M265)
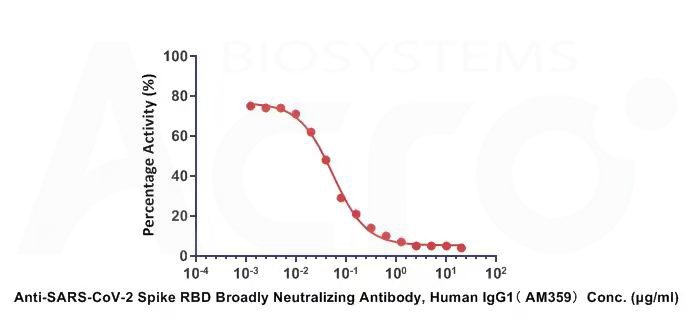
Anti-SARS-CoV-2 Spike RBD Broadly Neutralizing Antibody, Human IgG1(AM359)(Cat.No. SPD-M265) can neutralize SARS-CoV-2 (Omicron) Spike RBD by inhibiting RBD: ACE2 interaction. The ACE2-coated plate is incubated with the SARS-CoV-2 (Omicron) Spike RBD and treated with the neutralizing antibody at increasing concentration. The IC50 value is 0.05313ug/mL.
Antibodies
| Cat.No. | Source | Product description | Isotype | Epitope | Preorder/Order |
|---|---|---|---|---|---|
| SPD-M265 | Human | Anti-SARS-CoV-2 Spike RBD Broadly Neutralizing Antibody, Human IgG1 | Human IgG1 | Spike RBD | |
| S1N-M122 | Mouse | Anti-SARS-CoV-2 Spike RBD Neutralizing Antibody, Chimeric mAb, Human IgG1 (AM122) | Human IgG1 | Spike RBD | |
| S1N-M130 | Mouse | Anti-SARS-CoV-2 Spike RBD Antibody, Chimeric mAb, Human IgG1 (AM130) | Human IgG1 | Spike RBD |
Recombinant Antigens
| Molecule | Cat.No. | Tag | Mutation | Product description | Preorder/Order |
|---|---|---|---|---|---|
| Spike RBD | SPD-C522e | His Tag | G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H | SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) | |
| Spike RBD | SPD-C82E4 | His Tag & Avi Tag | G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H | Biotinylated SARS-CoV-2 Spike RBD, His,Avitag (B.1.1.529/Omicron) | |
| Spike protein | SPN-C52Hz | His Tag | A67V, HV69-70del, T95I, G142D, VYY143-145del, N211del, L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F | SARS-CoV-2 Spike Trimer, His Tag (B.1.1.529/Omicron) | |
| Spike protein | SPN-C82Ee | His Tag & Avi Tag | A67V, HV69-70del, T95I, G142D, VYY143-145del, N211del, L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F | Biotinylated SARS-CoV-2 Spike Trimer, His,Avitag (B.1.1.529/Omicron) | |
| Spike NTD | SPD-C522d | His Tag | A67V, HV69-70del, T95I, G142D, VYY143-145del, N211del, L212I, ins214EPE | SARS-CoV-2 Spike NTD, His Tag (B.1.1.529/Omicron) | |
| Spike S1 | S1N-C52Ha | His Tag | A67V, HV69-70del, T95I, G142D, VYY143-145del, N211del, L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H | SARS-CoV-2 Spike S1, His Tag (B.1.1.529/Omicron) | |
| Nucleocapsid protein | NUN-C52Ht | His Tag | P13L, ERS31-33del, R203K, G204R | SARS-CoV-2 Nucleocapsid protein, His Tag (B.1.1.529/Omicron) |
Antibody titer assay kits
| Cat.No. | Product Description | sample | Preorder/Order |
|---|---|---|---|
| RAS-N056 | Anti-SARS-CoV-2 (B.1.1.529) Neutralizing Antibody Titer Serologic Assay Kit (Spike RBD) | Human Serum | |
| RAS-T057 | Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG Titer Serologic Assay Kit (Spike RBD) | Human Serum | |
| RAS-T069 | Anti-SARS-CoV-2 (B.1.1.529) Total Antibody Titer Serologic Assay Kit (Spike RBD) | Human Serum | |
| RAS-T059 | Mouse Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG Titer Serologic Assay Kit (Spike RBD) | Mouse Serum | |
| RAS-T060 | Mouse Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG Titer Serologic Assay Kit (Spike Trimer) | Mouse Serum | |
| RAS-T061 | Mouse Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG Titer Serologic Assay Kit (Spike S1) | Mouse Serum | |
| RAS-T062 | Monkey Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG Titer Serologic Assay Kit (Spike RBD) | Monkey Serum | |
| RAS-T063 | Monkey Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG Titer Serologic Assay Kit (Spike Trimer) | Monkey Serum | |
| RAS-T064 | Monkey Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG Titer Serologic Assay Kit (Spike S1) | Monkey Serum | |
| RAS-T066 | Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG2 Titer Serologic Assay Kit (Spike RBD) | Human Serum | |
| RAS-T067 | Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG3 Titer Serologic Assay Kit (Spike RBD) | Human Serum | |
| RAS-T068 | Anti-SARS-CoV-2 (B.1.1.529) Antibody IgG4 Titer Serologic Assay Kit (Spike RBD) | Human Serum |
On November 25th, The National Institute for Communicable Diseases (NICD) in South Africa issued a statement that a new SARS-CoV-2 variant B.1.1.529 was detected in South Africa. The mutant strain has an astonishing 35 mutations on the spike protein, with at least 15 mutations in the RBD region, which plays an important role in the process of viral binding to host cells. The number of mutations found on this strain is much higher than that of the previously discovered mutant strains, thus alerting the global science community. On November 26th, the World Health Organization (WHO) listed B.1.1.529 as the fifth "Variant of Concern (VOC)” and named the mutant with the Greek letter Omicron.
The research on Omicron’s infectivity and immune escape ability is ongoing. Despite considerable uncertainties, the preliminary results are worrying:
Omicron may be more infectious than Delta
- The WHO revealed at a press conference that in the past two days, Omicron (B.1.1.529) has rapidly spread from 23 countries to 38 countries, especially in regions where it was first detected. In South Africa, Omicron is rapidly replacing Delta as the dominant viral strain. The French government's COVID-19 pandemic consultant predicts that by the end of January 2022, Omicron is likely to replace Delta as the main strain in France.
Omicron's immune escape ability is stronger than Beta and Delta
- The world's first study on Omicron mutant strains published by researchers from Stellenbosch University in South Africa showed that the risk of secondary infection caused by Omicron is three times that of Beta and Delta, indicating that the Omicron’s immune escape ability is stronger than Beta and Delta1.
Because most of the COVID-19 vaccines and therapeutic neutralizing antibodies are developed against the spike protein of the wild-type strain, such high numbers of mutations in the Omicron spike are likely to cause decrease in effectiveness, or even complete failure of most vaccines and therapeutic antibodies. Therefore, there is an urgent need to evaluate the protective efficacy of existing anti-viral products against Omicron and start to plan on new anti-Omicron strategies. On Nov 26th, Moderna has already announced the initiation of vaccine development program mRNA-1273.529 for Omicron, which they will advance to clinical stage 2 within 60-90 days2.
1. Increased risk of SARS-CoV-2reinfection associated with emergence of the Omicron variant in South Africa
2. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-strategy-address-omicron-b11529-sars-cov-2
This web search service is supported by Google Inc.
