1. Hugh Market.
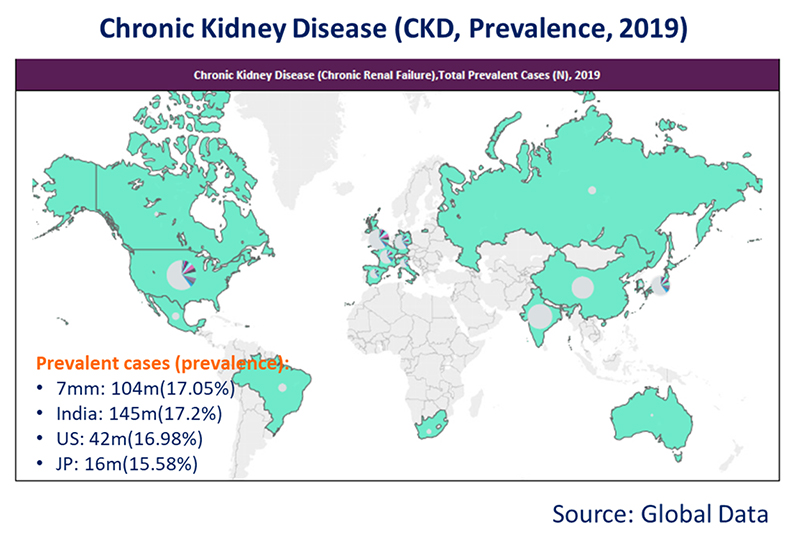
(1) CKD is a major public health problem across the world. India: 145m (17.2%), US: 42m (16.98%), JP: 16m (15.58%) people have CKD.
(2) 90% of EPO, a key factor for erythropoiesis, is made in kidney. Kidney damage leads to EPO deficiency and releases inflammatory factors that inhibit EPO secretion. Inflammation increases hepcidin which inhibits iron absorption and inhibits iron transport to the liver and spleen, leading to reduction of serum iron and finally reduction of HGB. Therefore 60%-80% of CKD patients combine with renal anemia.
2. Unmeet needs with current therapy
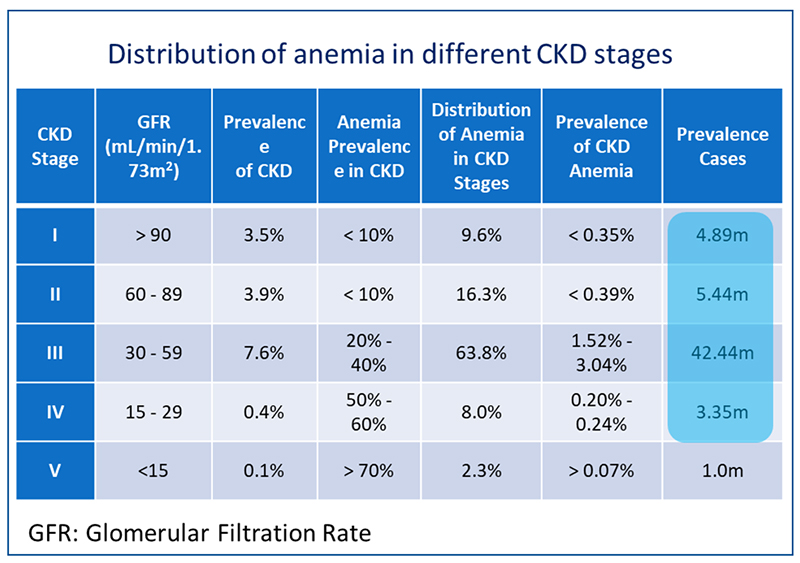
(1) rEPO+Chalybeate is current strategy for renal anemia. However, this therapy is mainly used for the treatment of dialysis patients and patients who are about to dialysis (CKD stage 5, taking up only 2.3% of CKD patients), and many non-dialysis patients (CKD stage 3-4, 71.8% of CKD patients) are lack of efficient therapies.
(2) Severe cardiovascular adverse reactions (hypertension, stroke) of rEPO with FDA black boxing warning. Poor response to rEPO therapy for 20% of patients with chronic inflammation.
(3) The expensive price and inconvenient administrate route of biologics.
3. HIF-PHs inhibitor is a potent solution for renal anemia.
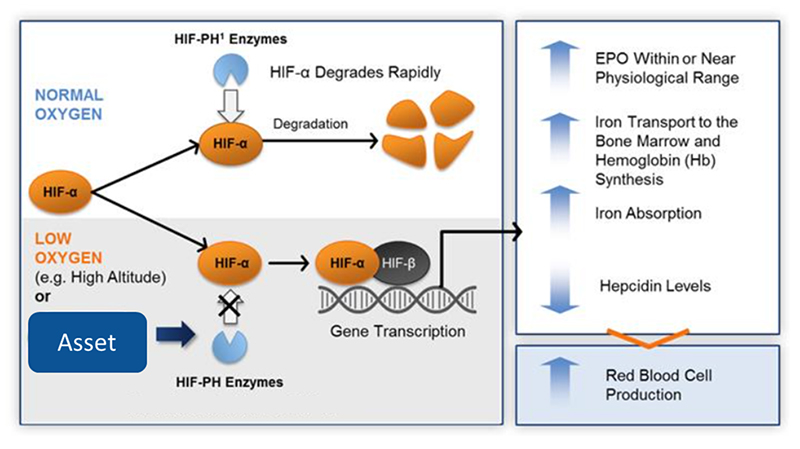
(1) The asset prevents HIF-1α degradation from HIF-PH enzymes, simulating the affair in hypoxia, which promotes EPO mRNA transcription and restore EPO secretion.
(2) HIF-PHs inhibition is thought to be a potent solution. Several HIF-PHs inhibitors have been approved in JP, India, AU, and China.
(3) This strategy not only work for renal anemia, but also have a potential to extend chemotherapy anemia and myelodysplastic syndrome (MDS) related anemia indications, which remains a much larger market size.
4. An effective PHD1/2/3 inhibitor.
The asset inhibits PHD1/2/3 activity at nanomolar level, slightly better than competitor FG-4592.
5. Great in vitro activity.
The asset is more effective than FG-4592 on inhibition of EPO secretion by inflammatory factors in Hep3B.
6. Excellent in vivo efficacy.
(1) The asset increased RBC and Hb in a dose-dependent manner in normal cynomolgus monkeys.
(2) The asset can improve the HGB level of model rats in a dose-dependent manner and no drug accumulation was observed after repeated administration.
7. Good safety in phase I trial.
(1) Plasma EPO reached maximum concentration 10-12 hour after dosing. And there was no accumulation of plasma EPO after repeated administration.
(2) After repeated administration of asset (40, 70, 100 mg), the reticulocyte count was significantly increased with the decrement of the hepcidin level, indicating that asset can effectively promote hematopoiesis and regulate iron metabolism.
(3) No severe or serious AEs were observed in this study. The incidence of adverse events was not significantly associated with dose escalation, indicating that asset is safe in healthy subjects.
1. Asset type: HIF-PHs inhibitor
2. Indication: CKD and Renal anemia
3. Modality: Small molecular, FIC
4. Patent: Applicated in 2015
5. Research phase: Phase 1
6. Cooperation demands: License-out or co-development of the global right (except for China)
7. Research progress:
(1) Phase 1 trials Ia is completed and patent has been applicated;
(2) Good DMPK and safety properties in preclinical study;
(3) Safety better than FG-4592;
(4) Potentially extend chemotherapy anemia and myelodysplastic syndrome (MDS) related anemia indications.

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.
To enable antibody characterization methodsACROBiosystems has developed a series of enzymes.such as ldeS, SpeB, EndoH, and Endo S proteases, toassist with the characterization of antibodies and theirrelated post-translational modifications (PTMs)

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
This web search service is supported by Google Inc.
