1. Superior Efficacy: Local injection increase TMZ concentration in the targeted brain tissue
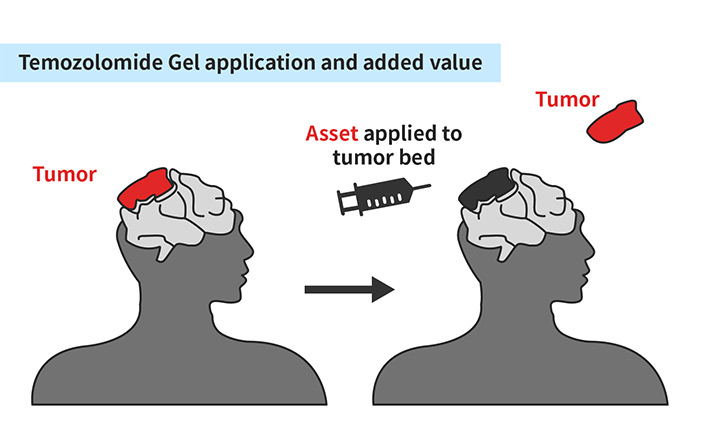
1) In a mouse glioblastoma model, the asset reduces tumor size even before starting radiation therapy and systemic TMZ administration.
2) Local injection of the novel formulation dramatically enhances tumor growth inhibition compared to radiation + systemic TMZ by 34%
2. Superior Safety: local injection limits unnecessary systemic exposure to TMZ
1) Gel formulation offers superior local concentration for tumor inhibition with decreased risk of systemic side effects.
2) TMZ plasma/brain ratios are only 0.1%–4% indicating retention of drug intracranially.
3. Superior MoA:
TMZ therapy is known to depend on the methylation status of the O6-methylguanine-DNA methyltransferase gene (MGMT) promoter, the current data shows that local application for the target asset is independent of the methylation status of the MGMT promoter, which provides better coverage of patients with different types of GBM.
1. Asset type: Viscous Gel Temozolomide
2. Indication: Glioblastoma
3. Modality: Small molecule
4. Research: Ready to start phase 1 (improved variant of Temodex®)
5. Demands: License-out or co-development
6. Research progress: Clinical trials completed in CIS region and registered as first-line treatment of glioblastoma
Research progress:
1) Clinical trials completed in CIS region and registered as first-line treatment of glioblastoma
2) Clinical trial data shows increased patient’s life for more than 9 months

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.
To enable antibody characterization methodsACROBiosystems has developed a series of enzymes.such as ldeS, SpeB, EndoH, and Endo S proteases, toassist with the characterization of antibodies and theirrelated post-translational modifications (PTMs)

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
This web search service is supported by Google Inc.
