1. Clear MOA
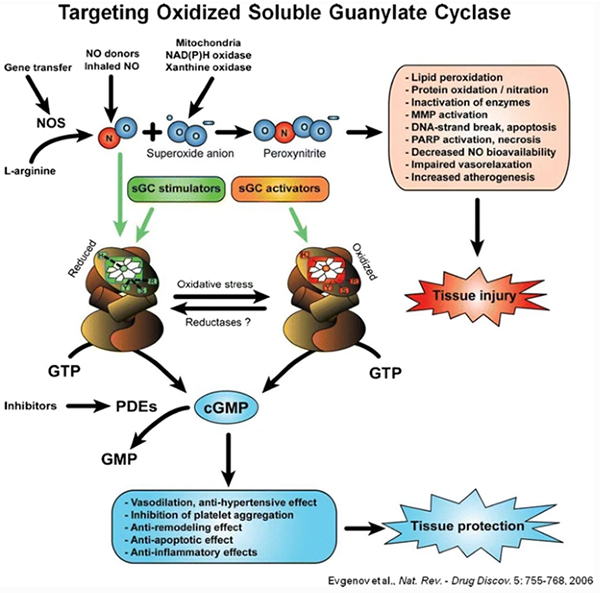
(1) sGC is an enzyme in the cardiopulmonary system that relaxes vascular smooth muscles, resulting in pulmonary vasodilation, reduced PAH, and improved cardiac output.
(2) Soluble guanylate cyclase (sGC) stimulators are medications used to treat PAH, CTEPH, HFrEF and CKD.
2. High efficacy in vitro.
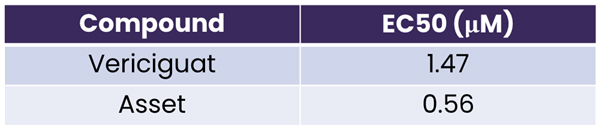
(1) The asset is better than 2nd generation of sGC stimulator Vericiguat.
(2) Asset has a desirable selectivity. The IC50 values were 7.74 μM for human PPARγ, 3.05 μM for human 5-HT2B and 5.79 μM for rat Sodium Channel, Site 2, corresponding to 1187-times, 467-times, and 888-times.
3. Better efficacy in vivo.
(1) Improved MCT-induced increase in RVSP.
(2) Prevented the increases in LVSP and LVEDP on abdominal aortic constriction-induced cardiac pressure overload in rats.
(3) Improved the survival rate and GSI (glomerulosclerosis index) in the diabetic nephropathy model.
4. The asset is not an inhibitor of CYPIA2, 2B6, 2C8, 2C19, 2D6 or 3A4 and is not an inducer of CYP1A2, 2B6, or 3A4. It is a substrate of P-gp but is not a substrate of BCRP.
5. Good safety.
(1) No effects were noted on the central nervous systems (30 mpk in rats) and respiratory function (10 mpk in dogs).
(2) No mutagenic and chromosome aberration risk.
(3) Well-tolerated in single-dose toxicity after PO to rats (≤ 400 mpk) or dogs (≤ 2000 mpk).
6. Great Phase 1 data: well tolerated, the half-life indicated suitability for once-daily dosing.
1. Asset type: sGC stimulator
2. Indication: Pulmonary arterial hypertension (PAH), Chronic thromboembolic pulmonary hypertension (CTEPH), Heart failure with reduced ejection fraction (HFrEF), Chronic kidney disease (CKD)
3. Modality: Small molecular
4. Research phase: Phase 1, IP has been granted in US, AU, CN
5. Cooperation demands: License-out or co-development
6. Research progress:
(1) Phase 1 trial is ongoing.
(2) The GSI after asset administration is lower than that of Losartan.

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.
To enable antibody characterization methodsACROBiosystems has developed a series of enzymes.such as ldeS, SpeB, EndoH, and Endo S proteases, toassist with the characterization of antibodies and theirrelated post-translational modifications (PTMs)

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
This web search service is supported by Google Inc.
