 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
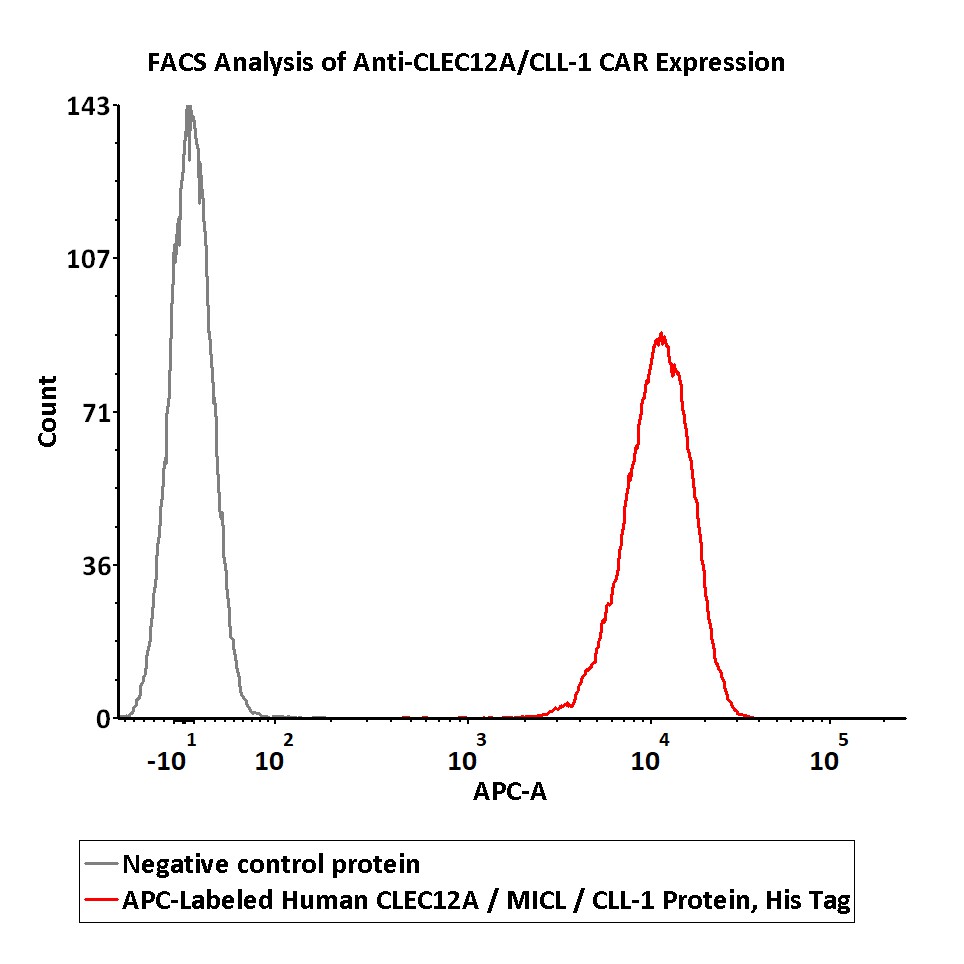
5e5 of anti-CLEC12A / CLL-1 CAR-293 cells were stained with 100 μL of 1:25 dilution (4 μL stock solution in 100 μL FACS buffer) of APC-Labeled Human CLEC12A, His Tag (Cat. No. CLA-HA248) and negative control protein respectively. APC signal was used to evaluate the binding activity (QC tested).

5e5 of anti-CLEC12A/CLL-1 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CLEC12A, His Tag (Cat. No. CLA-HP2Q3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).

Immobilized Biotinylated Human CLEC12A Protein, His,Avitag, premium grade (Cat. No. CLA-H82E6) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate, can bind Monoclonal Anti-Human CLEC12A Antibody, Human IgG1 with a linear range of 0.1-0.8 ng/mL (QC tested).
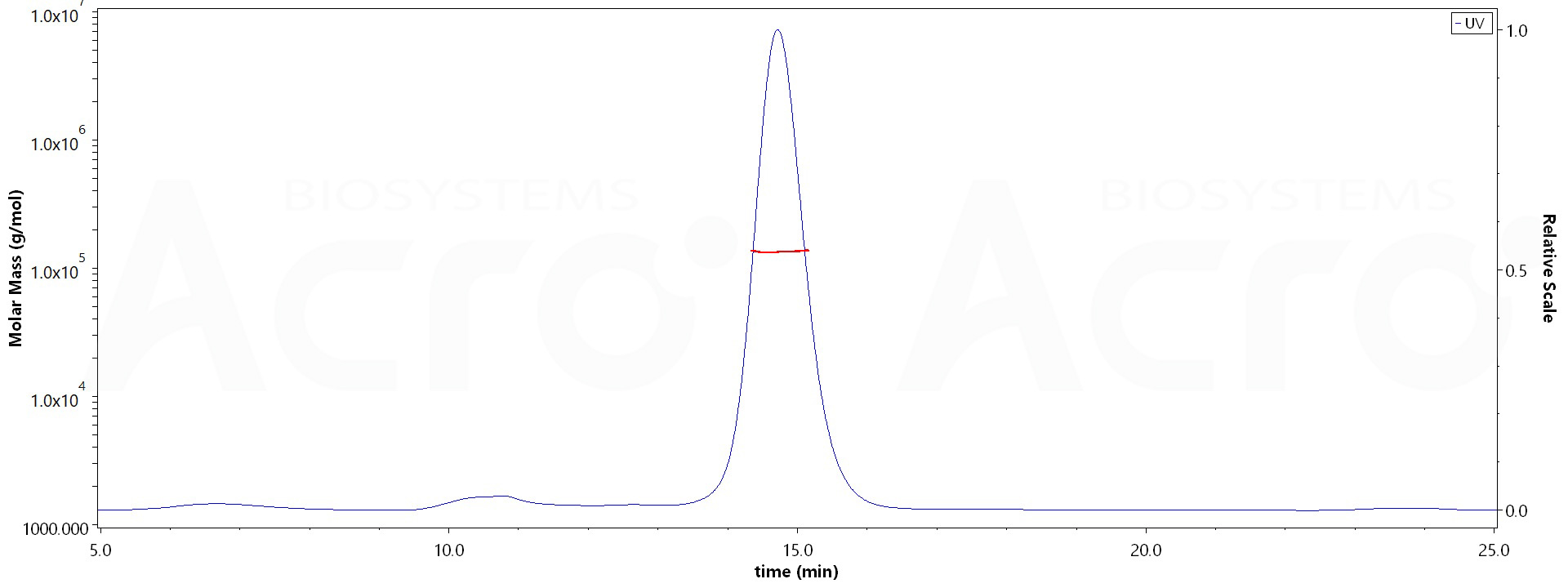
The purity of Human CLEC12A, Fc Tag (Cat. No. CLA-H5266) is more than 90% and the molecular weight of this protein is around 125-140 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ICG-136 | ICG-136; 123b-33bcCAR | Phase 1 Clinical | Leukemia, Myeloid, Acute | Details | |
| CD123/CLL1 CAR-T Cell Therapy (Fujian Medical University) | Fujian Medical University | Details | |||
| CLL1 CAR-T(Zhejiang University) | Phase 1 Clinical | Zhejiang University | Leukemia, Myeloid, Acute | Details | |
| CLL1+CD33 CAR-T(Zhejiang University) | Phase 1 Clinical | Zhejiang University | Leukemia, Myeloid, Acute | Details | |
| CLL1-CD33 cCART cell therapy (iCell Gene Therapeutics) | CLL1-CD33-cCAR | Phase 1 Clinical | Chengdu Military General Hospital | Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Hematologic Neoplasms; Myelodysplastic Syndromes; Myeloproliferative Disorders; Leukemia, Myeloid, Acute | Details |
| CLL1 CAR T-cell Therapy(Yake Biotechnology) | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd | Leukemia, Myeloid, Acute | Details | |
| Anti-CLL1 CAR T cell therapy(Soochow University) | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University | Leukemia, Myeloid, Acute | Details | |
| CLL-1.CAR T cell therapy (Baylor College of Medicine) | Phase 1 Clinical | Baylor College Of Medicine | Leukemia, Myeloid, Acute | Details | |
| Anti-CD33/CLL1 CAR-NK Cell Therapy | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Leukemia, Myeloid, Acute | Details | |
| KITE-222 | Phase 1 Clinical | Kite Pharma | Leukemia, Myeloid, Acute | Details | |
| Anti-CD33/CLL-1 CAR-T (Legend) | Phase 1 Clinical | Legend Biotech Corp | Leukemia, Promyelocytic, Acute | Details | |
| Tepoditamab | MCLA-117 | Phase 1 Clinical | Pharmaceutical Research Associates, Institute Gustave-Roussy, Merus Nv, Vu University Medical Center, Lgc | Leukemia, Myeloid, Acute | Details |
This web search service is supported by Google Inc.
