 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!

5e5 of anti-CD4 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD4, His Tag (Cat. No. CD4-HP2E3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).

Immobilized Ibalizumab at 1 μg/mL (100 μL/well) can bind FITC-Labeled Human CD4, His Tag (Cat. No. CD4-HF2H7) with a linear range of 0.01-0.156 μg/mL (QC tested).
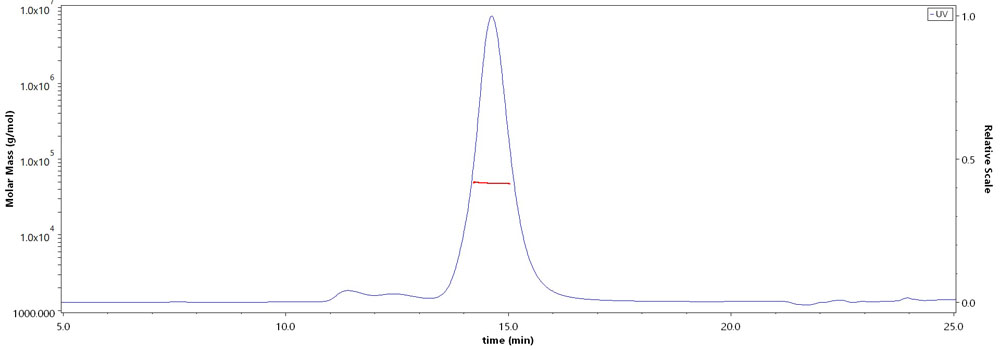
The purity of Biotinylated Rhesus macaque CD4,His,Avitag (Cat. No. CD4-R82E3) is more than 90% and the molecular weight of this protein is around 43-53 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ibalizumab | Hu5A8; TMB-355; TNX-355; 5AB | Approved | Biogen Inc | 特罗格佐, Trogarzo | EU | HIV Infections | Theratechnologies Europe Ltd | 2018-03-06 | HIV Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| CALRLong36 peptide (Herlev Hospital) | Phase 1 Clinical | Herlev Hospital | Myeloproliferative Disorders | Details | |
| CD4^LVFOXP3 Treg-like cell therapy | CD4^LVFOXP3 | Phase 1 Clinical | Stanford University | Polyendocrinopathies, Autoimmune | Details |
| TMB-365 | TMB-365 | Taimed Biologics Inc | Details | ||
| SCRI-E2CAR_EGFRtv1 | SCRI-E2CAR_EGFRtv1 | Umoja BioPharma Inc | Details | ||
| IMCY-0141 | IMCY-0141 | Imcyse Sa | Details | ||
| Anti-CD4 CAR T-cell therapy (University of Pennsylvania) | CAR-C34ZFN | University Of Pennsylvania | Details | ||
| RB-0003 | RB-0003; BNT-111 | Biontech Se, Tron | Details | ||
| Tregalizumab | hB-F5; BT-061 | Phase 2 Clinical | Biotest Pharma Gmbh | Drug Hypersensitivity; Arthritis, Rheumatoid; Psoriasis; Asthma | Details |
| Mosedipimod | EC-18 | Phase 2 Clinical | Enzychem Lifesciences Co | Neutropenia; Coronavirus Disease 2019 (COVID-19); Stomatitis; Febrile Neutropenia | Details |
| IT-1208 (Kyowa Hakko Kirin) | IT-1208 | Phase 1 Clinical | Kyowa Hakko Kirin Co Ltd | Neoplasms | Details |
| GEN-009 | GEN-009 | Phase 2 Clinical | Genocea Biosciences Inc | Skin Melanoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung | Details |
| VRC-07-523 | VRC-07-523-L S; VRC-HIVMAB075-00-AB; VRC-HIVMAB-075 -00-AB; VRC-07-523; TMB-380 | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), Taimed Biologics Inc | HIV Infections; Acquired Immunodeficiency Syndrome; Retroviridae Infections; Sexually Transmitted Diseases, Viral | Details |
| VRC-01-LS | VRC-HIVMAB-080-00-AB; VRC-01-LS | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| 10E8.4/iMab (Aaron Diamond AIDS Research Center) | TMB-370 | Phase 1 Clinical | Aaron Diamond Aids Research Center For The City Of New York, Inc | HIV Infections | Details |
| T-allo-10 | T-allo-10 | Phase 1 Clinical | Stanford University | Hematologic Diseases; Graft vs Host Disease | Details |
| CD4 CAR T cell therapy (iCell Gene Therapeutics) | Phase 1 Clinical | Lymphoma, T-Cell | Details | ||
| CD4-directed chimeric antigen receptor engineered T-cells (Icell Gene) | CD4CAR (Icell Gene) | Phase 1 Clinical | Stony Brook University School Of Medicine, Icell Gene Therapeutics (Int'L) Ltd, University Of Louisville | Lymphoma, T-Cell; Leukemia, T-Cell | Details |
| Autologous Regulatory Т-cell Therapy | Phase 2 Clinical | Institute Of Biophysics And Cell Engineering Of National Academy Of Sciences Of Belarus | Scleroderma, Systemic | Details | |
| VRC-HIVMAB091-00-AB | N6LS; Z258‐N6LS; VRC-HIVMAB091-00-AB | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| LB-1901 | LB-1901; LB1901 | Phase 1 Clinical | Nanjing Legend Biotechnology Co Ltd | Lymphoma, T-Cell, Peripheral; Lymphoma, T-Cell; Lymphoma, T-Cell, Cutaneous | Details |
| VRC-HIVMAB0115-00-AB | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details | |
| Semzuvolimab | dB4; UB-421; mAb-B4 | Phase 3 Clinical | United Biomedical Inc | HIV Infections | Details |
| ITV 1(Nonindustrial source) | ITV-1 | Phase 3 Clinical | Synexa Life Sciences, Immunotech Laboratories, Nonindustrial Source | HIV Infections | Details |
This web search service is supported by Google Inc.
