 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!

2e5 of anti-CD30 CAR-293 cells were stained with 100 μL of 1 μg/mL of FITC-Labeled Human CD30, His Tag (Cat. No.CD0-HF2H4) and negative control protein respectively, FITC signal was used to evaluate the binding activity (QC tested).
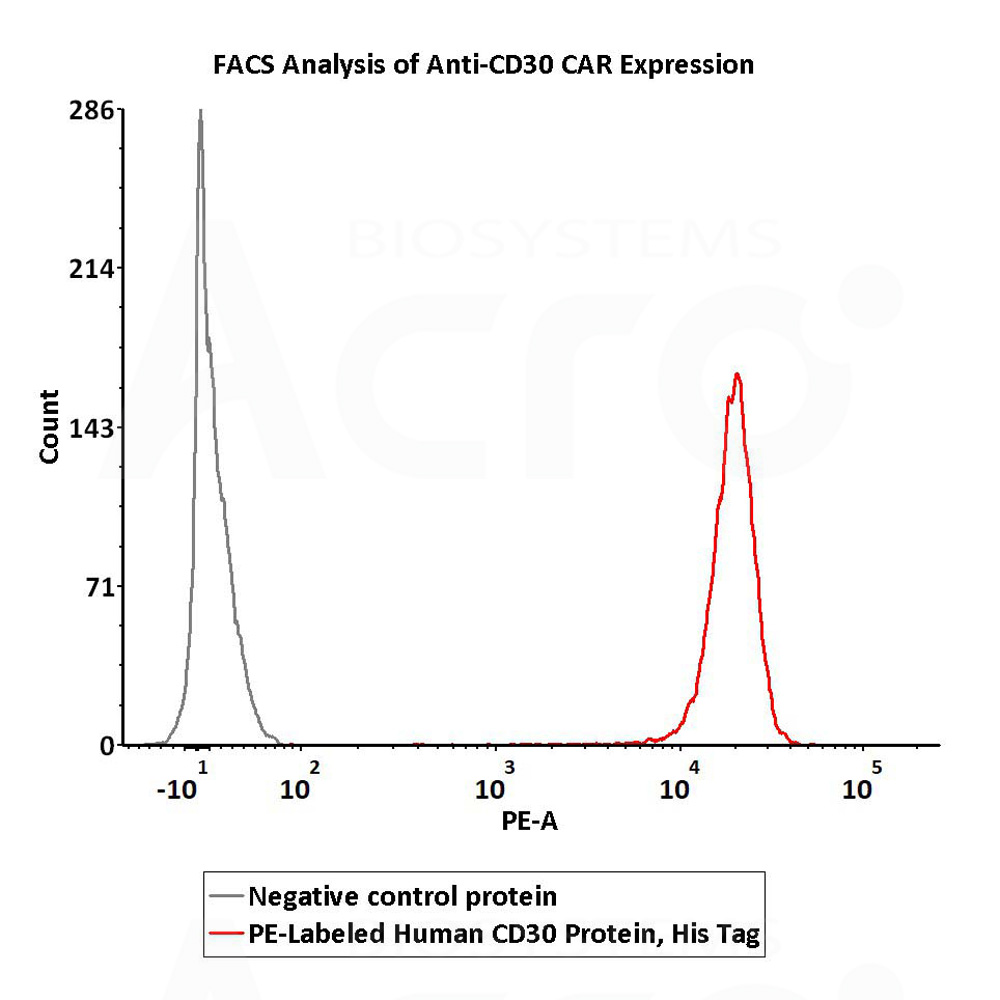
5e5 of anti-CD30 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD30, His Tag (Cat. No. CD0-HP2E3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
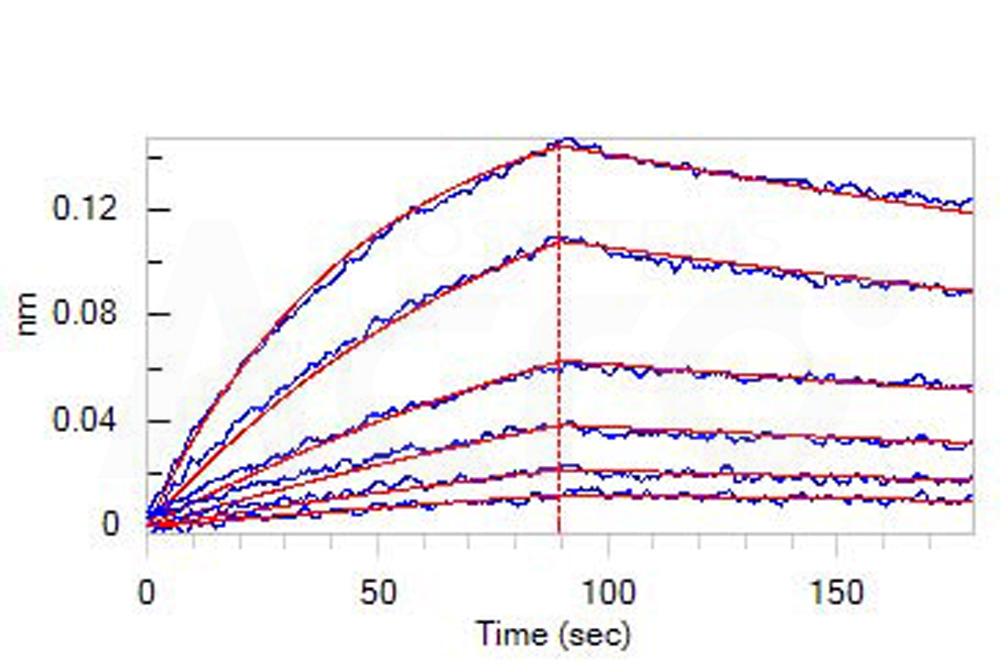
Loaded Human CD30 Ligand, His Tag (Cat. No. CDL-H524b) on HIS1K Biosensor, can bind Human CD30 Protein, Llama IgG2b Fc Tag (Cat. No. TN8-H5250) with an affinity constant of 55.5 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Brentuximab vedotin | SGN-30; SGN-35; cAC-10; SGD-1010; cAC10-vcMMAE; cAC10-Val-Cit-MMAE | Approved | Millennium Pharmaceuticals Inc, Seattle Genetics Inc | 安适利, Adcetris | Mainland China | Lymphoma, Primary Cutaneous Anaplastic Large Cell; Mycosis Fungoides | Takeda Pharma A/S | 2011-08-19 | Mesothelioma; Neoplasms, Germ Cell and Embryonal; Sarcoma, Kaposi; Lymphomatoid Papulosis; Mycosis Fungoides; Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Scleroderma, Diffuse; Lymphoma, T-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma, Non-Hodgkin; Sezary Syndrome; Lupus Erythematosus, Systemic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Primary Cutaneous Anaplastic Large Cell; Lymphoma, T-Cell, Peripheral; Leukemia, Mast-Cell; Enteropathy-Associated T-Cell Lymphoma; Lymphoma, Large B-Cell, Diffuse; Myelodysplastic Syndromes; Mastocytosis, Systemic; Scleroderma, Systemic; Graft vs Host Disease; Neoplasms; Hodgkin Disease; Carcinoma; Anemia, Refractory, with Excess of Blasts; Lymphoma, B-Cell; HIV Infections; Hematologic Diseases; Solid tumours | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Anti-CD30 CAR-T cell therapy (National Cancer Institute) | Hu30-CD28z | Phase 1 Clinical | National Cancer Institute | Lymphoma | Details |
| Anti CD30 CAR T Cell Therapy (First Song Therapeutics) | Zhejiang University | Details | |||
| MDX-1401 | MDX-1401 | Medarex | Details | ||
| Recombinant chimeric anti-CD30 monoclonal antibody-MCC-DM1 | F0002-ADC; B-006 | Phase 1 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd, Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Lymphoma, T-Cell, Peripheral; Hematologic Neoplasms | Details |
| Itezocabtagene autoleucel | TT-11 | Phase 2 Clinical | Tessa Therapeutics Ltd | Lymphoma, T-Cell, Peripheral; Lymphoma, B-Cell; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic | Details |
| CD30.CAR | H-27721; CD30.CAR | Phase 1 Clinical | Baylor College Of Medicine | Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| Anti-CD30 chimeric antigen receptor T cell therapy (Immune cell) | Phase 1 Clinical | Immune Cell Inc | Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details | |
| SGN-CD30C | SGN-CD30C | Phase 1 Clinical | Seattle Genetics Inc | Lymphoma | Details |
| Anti-CD30 CAR T-cell therapy (Wuhan Bio-Raid) | Phase 1 Clinical | Wuhan Bio-Raid Biotechnology | Lymphoma, T-Cell, Peripheral; Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Extranodal NK-T-Cell; Lymphoma, T-Cell; Lymphoma, Large-Cell, Anaplastic | Details | |
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Pancreatic Neoplasms; Lymphoma, Non-Hodgkin | Details | |
| EBV-specific-CAR.CD30 | EBV-specific-CAR.CD30; CAR.CD30 EBV-specific-CTLs | Phase 1 Clinical | Baylor College Of Medicine, Texas Children'S Hospital, Methodist Hospital System | Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| CD30.CAR-EBVST cell therapy (Baylor College of Medicine) | TT-11X | Phase 1 Clinical | Baylor College Of Medicine, Tessa Therapeutics Ltd | Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Lymphoma, Extranodal NK-T-Cell | Details |
| AFM-13 | AFM-13 | Phase 2 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Mycosis Fungoides | Details |
| HSP-CAR-30 | HSP-CAR-30 | Phase 2 Clinical | Fundació Institut De Recerca De L | Hodgkin Disease; Lymphoma, T-Cell | Details |
| Anti-CD30 CAR T-cell therapy (General Hospital of the People's Liberation Army/Cellular Biomedicine) | CAR30-NKT; CBM-C30.1; CD30ScFv-CD8-CD137-CD3zeta | Phase 2 Clinical | Pla General Hospital | Hodgkin Disease | Details |
| PRA-052 | PRA-052 | Phase 1 Clinical | Prometheus Biosciences Inc | Colitis, Ulcerative | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Solid tumours; Chondrosarcoma; Leukemia; Neoplasms; Hodgkin Disease; Neuroblastoma; Lymphoma | Details | |
| ATLCAR.CD30 cells (UNC Lineberger Comprehensive Cancer Center) | Phase 2 Clinical | Unc Lineberger Comprehensive Cancer Center | Lymphoma, T-Cell, Peripheral; Lymphatic Diseases; Immunoproliferative Disorders; Hodgkin Disease; Neoplasms; Immune System Diseases; Lymphoproliferative Disorders; Lymphoma, Non-Hodgkin; Lymphoma; Neoplasms, Germ Cell and Embryonal | Details |
This web search service is supported by Google Inc.
