 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| AXL inhibitor | Small molecule | Oncology/Cancer | STK11_mut NSCLC,other cancers | Phase I | Global |
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
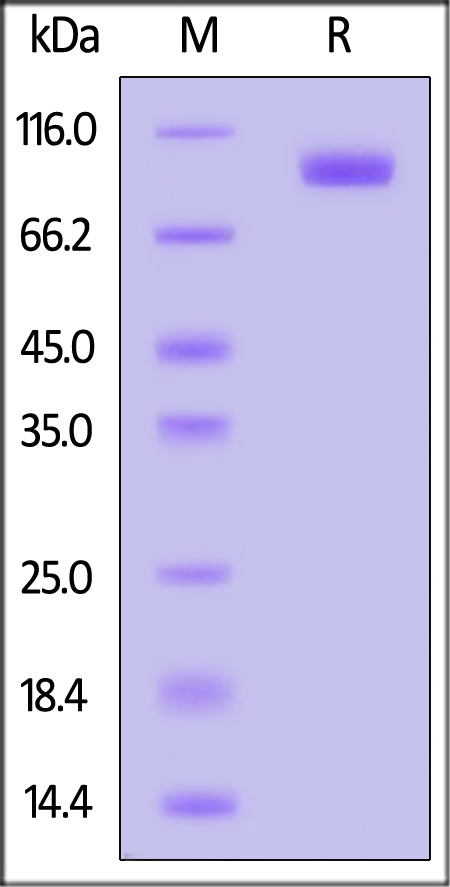
| AXL-H82F9 | Human | Biotinylated Human AXL Protein, Fc,Avitag™ (MALS verified) |  |
|

|
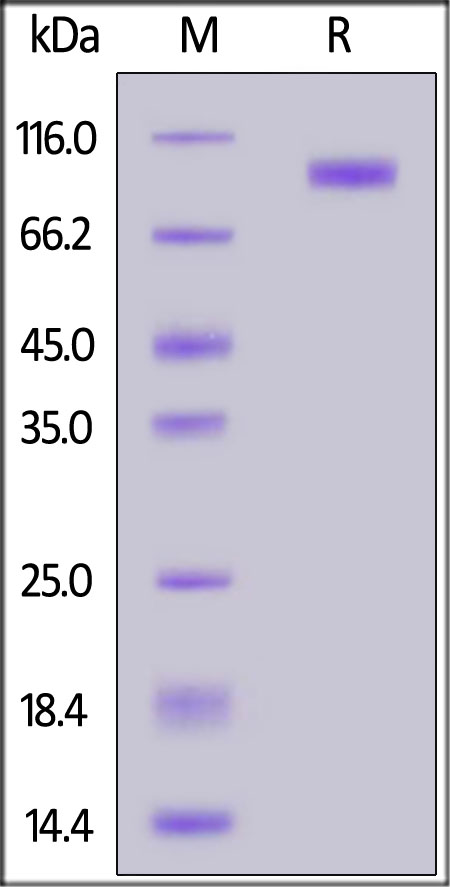
| AXL-H5253 | Human | Human Axl Protein, Fc Tag (MALS verified) |  |
|

|
| AXL-C52H3 | Cynomolgus | Cynomolgus Axl Protein, His Tag |  |

|
|
| AXL-H5226 | Human | Human Axl Protein, His Tag |  |

|

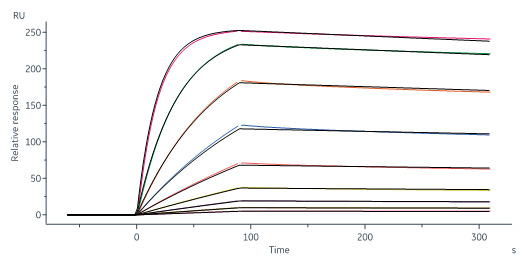
Human Axl, Fc Tag (Cat. No. AXL-H5253) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human GAS6, His Tag (Cat. No. GA6-H5249) with an affinity constant of 1.23 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Gilteritinib Fumarate | ASP-2215; 66D-92MGC8M (UNII code); ASP-2215 hemifumarate | Approved | Astellas Pharma Inc | Xospata | Mainland China | Leukemia, Myeloid, Acute | Astellas Pharmaceutical (China) Co Ltd | 2018-09-21 | Solid tumours; Renal Insufficiency; Hepatic Insufficiency; Leukemia, Myeloid, Acute; Carcinoma, Non-Small-Cell Lung | Details |
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | Japan | Carcinoma, Renal Cell | Takeda | 2012-11-29 | Osteosarcoma; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Peritoneal Neoplasms; Bile Duct Neoplasms; Hepatic Insufficiency; Astrocytoma; Breast Neoplasms; Sarcoma, Ewing; Lymphoma; Carcinoma, Adenosquamous; Adenocarcinoma, Clear Cell; Sarcoma, Clear Cell; Medullary thyroid cancer (MTC); Cholangiocarcinoma; Prostatic Neoplasms; Neurofibroma, Plexiform; Urethral Neoplasms; Carcinoma, Neuroendocrine; Meningioma; Carcinoma, Non-Small-Cell Lung; Paraganglioma; Melanoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Adenocarcinoma; Neoplasms, Germ Cell and Embryonal; Carcinoma, Hepatocellular; Neuroblastoma; Glioma; Leukemia, Myeloid, Acute; Carcinoma, Squamous Cell; Endometrial Neoplasms; Lung Neoplasms; Sarcoma, Alveolar Soft Part; Uterine Neoplasms; Fallopian Tube Neoplasms; Carcinoid Tumor; Neurofibromatoses; Seminoma; Carcinoma, Transitional Cell; Glioblastoma; Rejection of liver transplantation; Carcinoma, Merkel Cell; Hepatoblastoma; Pain; Pheochromocytoma; Neoplasms; Carcinoma, Renal Cell; B | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| NP-01 (Shijiazhuang No.4 Pharmaceutical/Nanjing Nadingfei Medical Technology) | NP-01 | Phase 1 Clinical | Shijiazhuang No 4 Pharmaceutical Co Ltd, Nanjing Nadingfei Pharmaceutical Technology Co Ltd | Liver Neoplasms; Solid tumours; Stomach Neoplasms; Lung Neoplasms | Details |
| AGX-0073 | AGX-0073 | Phase 1 Clinical | Shanghai Aojian Biological Technology Co Ltd | Solid tumours | Details |
| NP-107 | NP107; NP-107 | Phase 1 Clinical | Shanghai Nawei Biotechnology Co Ltd | Solid tumours | Details |
| BPI-9016 | BPI-9016; BPI-9016M | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| ONO-7475 | ONO-7475 | Phase 2 Clinical | Ono Pharmaceutical Co Ltd | Leukemia, Myeloid, Acute | Details |
| Tilvestamab | BGB-149 | Phase 1 Clinical | Bergenbio | Ovarian Neoplasms | Details |
| Enapotamab vedotin | AXL-107-MMAE | Phase 2 Clinical | Seattle Genetics Inc, Genmab A/S | Ovarian Neoplasms; Sarcoma; Endometrial Neoplasms; Thyroid Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma | Details |
| XZB-0004 | SLC-391; XZB-0004 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Foodborne Diseases; Carcinoma, Non-Small-Cell Lung | Details | |
| Butylidenephthalide | HK-001; LF-001 (Everfront Biotech); NSC-325307; BDPH | Phase 2 Clinical | Everfront Biotech Co Ltd | Glioma; Amyotrophic Lateral Sclerosis | Details |
| Glesatinib | 7Q29OXD98N; MG-90265H9; MG-90265gly; MG-90265; MG-90265X; MGCD-265 | Phase 2 Clinical | Mirati Therapeutics Inc | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| INCB-081776 | INCB-81776; INCB-081776 | Phase 1 Clinical | Incyte Corp | Solid tumours; Neoplasms | Details |
| Dubermatinib | TP-0903 | Phase 2 Clinical | Sumitomo Dainippon | Ovarian Neoplasms; Solid tumours; Neoplasms; Colorectal Neoplasms; Leukemia, Myeloid, Acute; Leukemia, Lymphocytic, Chronic, B-Cell; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Bemcentinib | R-428; BGB-324 | Phase 2 Clinical | Rigel Pharmaceuticals Inc | Coronavirus Disease 2019 (COVID-19); Inflammatory Breast Neoplasms; Triple Negative Breast Neoplasms; Myelodysplastic Syndromes; Adenocarcinoma of Lung; Pancreatic Neoplasms; Mesothelioma; Leukemia, Myeloid, Acute; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| KC1036 | KC1036; KC-1036 | Phase 2 Clinical | Beijing Konruns Pharmaceutical Co Ltd | Solid tumours; Hematologic Neoplasms; Digestive System Neoplasms; Neoplasm Metastasis | Details |
| Q-702 | Q-702 | Phase 2 Clinical | Qurient Co Ltd | Solid tumours; Liver Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Neoplasm Metastasis; Uterine Cervical Neoplasms | Details |
| FC-084-CSA | FC-084-CSA; FC084CSA | Phase 1 Clinical | Zhongshan Yinuo Weishen New Drug Research and Development Co Ltd | Solid tumours | Details |
| PF-07265807 | PF-07265807 | Phase 1 Clinical | Solid tumours; Neoplasm Metastasis | Details | |
| TT-00973 | TT-00973 | Phase 1 Clinical | TransThera Sciences (Nanjing) Inc | Solid tumours | Details |
| HH-30134 | HH-30134 | Phase 1 Clinical | ShangHai HaiHe Biopharma Co Ltd | Solid tumours; Neoplasms | Details |
| Ningetinib Tosylate | CT-053-PTSA; CT-053 | Phase 2 Clinical | Hec Pharm Co Ltd | Solid tumours; Intestinal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Lung Neoplasms; Leukemia, Myeloid, Acute; Carcinoma, Non-Small-Cell Lung | Details |
| CCT301-38 | CCT301-38; CCT301-38 AXL | Phase 2 Clinical | F1 Oncology, Bioatla | Carcinoma, Renal Cell; Rhinitis, Allergic, Seasonal | Details |
| Mipasetamab uzoptirine | ADCT-601; BGB-601 | Phase 1 Clinical | Adc Therapeutics Sa | Solid tumours; Neoplasms | Details |
| Mecbotamab vedotin | BA-3011; BA3011; BA 3011 | Phase 2 Clinical | Bioatla | Solid tumours; Leiomyosarcoma; Ovarian Neoplasms; Sarcoma, Synovial; Liposarcoma; Sarcoma; Osteosarcoma; Sarcoma, Ewing; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
This web search service is supported by Google Inc.
