| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| EGFR inhibitor | Small molecule | Oncology/Cancer | Esophageal Carcinoma Pancreatic Cancer | Phase III | Global |
| New Generation, Targeting Rare and Refractory Mutations of EGFR | Small molecule | Oncology/Cancer | Non-small cell lung cancer | Phase I | Global |
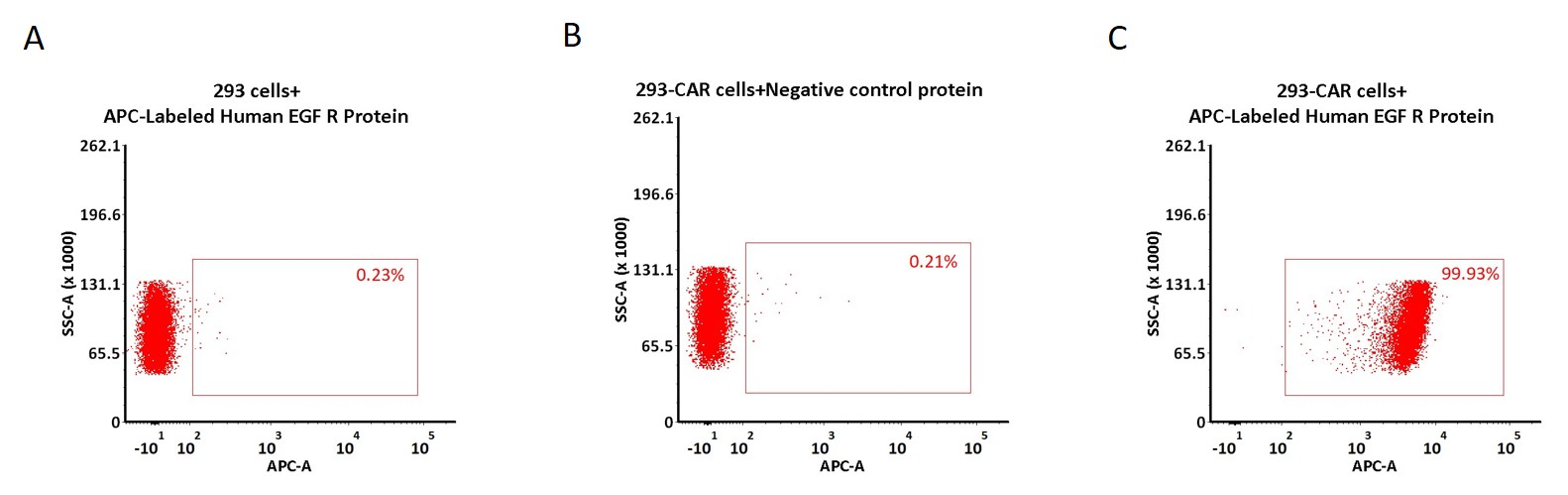
5e5 of anti-EGFR CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of APC-Labeled Human EGF R Protein, His Tag (Cat. No. EGR-HA2H8) and negative control protein respectively (Fig. C and B), and non-transfected 293 cells were used as a control (Fig. A). APC signal was used to evaluate the binding activity (QC tested).
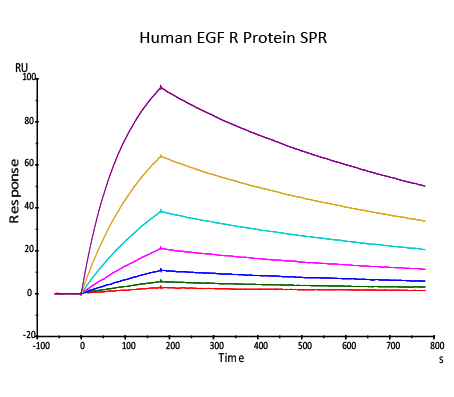
Immobilized Erbitux on CM5 Chip via anti-human Fc IgG, can bind Human EGF R, His Tag, low endotoxin (Cat.No. EGR-H522a) with an affinity constant of 0.492 nM as determined in SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse epidermal growth factor (Hangzhou Tianmushan Pharmaceutical) | Approved | Hangzhou Tianmushan Pharmaceutical Enterprise Co Ltd | 一夫 | Wounds and Injuries | Details | |||||
| Recombinant epidermal growth factor drop (Pavay Gene Pharmaceutical) | Approved | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 易贝 | Mainland China | Corneal Diseases | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 2002-04-12 | Corneal Diseases; Xerophthalmia | Details | |
| Recombinant epidermal growth factor (Shanghai Haohai ) | Approved | Shanghai Haohai Biological Technology Co Ltd | 康合素 | Mainland China | Burns | Shanghai Haohai Biological Technology Co Ltd | 2001-01-01 | Burns | Details | |
| Cetuximab biosimilar (CinnaGen) | Approved | Cinnagen | Iran | Colorectal Neoplasms | Cinnagen | 2017-01-01 | Colorectal Neoplasms | Details | ||
| Recombinant Human Epidermal Growth Factor Derivative Eye Drops (Shenzhen Wastin Genetech) | Approved | Shenzhen Watsin Genetech Ltd | 金因舒, GeneSoft | Mainland China | Corneal Diseases | Shenzhen Watsin Genetech Ltd | 2004-01-21 | Corneal Diseases | Details | |
| Recombinant human EGF conjugated vaccine | Approved | Biotech Pharmaceuticals Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | ||||||
| Recombinant Human Epidermal Growth Factor Derivative (Shenzhen Wastin Genetech) | rEGF | Approved | Shenzhen Watsin Genetech Ltd | 金因肽, GeneTime | Mainland China | Wounds and Injuries | Shenzhen Watsin Genetech Ltd | 2001-01-01 | Wounds and Injuries | Details |
| Recombinant epidermal growth factor gel (Pavay Gene Pharmaceutical) | Approved | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 易孚 | Mainland China | Skin Ulcer; Burns | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 2002-12-23 | Skin Ulcer; Burns | Details | |
| Epidermal growth factor biosimilar (RAS Lifesciences) | Approved | Ras Lifesciences | India | Ras Lifesciences | 2012-10-01 | Details | ||||
| Recombinant epidermal growth factor biosimilar (Elea) | r-hu-EGF | Approved | Elea | Argentina | Diabetic Foot; Burns | Elea | 2015-01-01 | Diabetic Foot; Burns | Details | |
| Recombinant epidermal growth factor (Bharat Biotech) | REGEN-D 60; REGEN-D 150 | Approved | Bharat Biotech International Ltd | India | Diabetic Foot; Burns | Bharat Biotech International Ltd | 2005-01-01 | Diabetic Foot; Burns | Details | |
| Recombinant epidermal growth factor (Center for Genetic Engineering and Biotechnology/Praxis Pharmaceuticals) | Approved | Praxis Pharmaceuticals, Center For Genetic Engineering And Biotechnology | Heberprot-P | Cuba | Diabetic Foot | null | 2007-09-01 | Diabetic Foot | Details | |
| PX-070101 | PX-070101 | Approved | Praxis Pharmaceuticals | Colombia | Diabetic Foot | Praxis Pharmaceuticals | 2015-07-01 | Diabetic Foot | Details | |
| Nepidermin | DWP-401 | Approved | Daewoong Pharmaceutical Co Ltd | Easyef | Egypt | Alopecia; Diabetic Foot | Daewoong Pharmaceutical Co Ltd | 2001-01-01 | Diabetic Foot; Alopecia; Dry Eye Syndromes | Details |
| Cetuximab sarotalocan | RM-1929; ASP-1929 | Approved | Aspyrian Therapeutics | Akalux | Japan | Head and Neck Neoplasms | Rakuten Medical Inc | 2020-09-25 | Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck | Details |
| Icotinib Hydrochloride | BPI-2009H; BPI-2009C | Approved | Betta Pharmaceuticals Co Ltd | 凯美纳, Conmana | Mainland China | Carcinoma, Non-Small-Cell Lung | Betta Pharmaceuticals Co Ltd | 2011-06-07 | Breast Neoplasms; Neuroma, Acoustic; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Brain metastases; Esophageal adenocarcinoma; Lung Neoplasms; Carcinoma, Adenosquamous; Solid tumours; Nasopharyngeal Carcinoma; Psoriasis; Adenocarcinoma of Lung; Neurofibromatosis 2; Bronchial Neoplasms; Esophageal Neoplasms; Stomach Neoplasms | Details |
| Dacomitinib | PF-299; PF-804; PF-299804; PF-00299804; PF-00299804-3; PF-00299804-03 | Approved | Pfizer Inc | Vizimpro | Mainland China | Carcinoma, Non-Small-Cell Lung | Pfizer Europe Ma Eeig | 2018-09-27 | Colorectal Neoplasms; Adenocarcinoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Penile Neoplasms; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Mouth Neoplasms; Head and Neck Neoplasms; Brain Neoplasms; Carcinoma, Large Cell; Liver Diseases; Glioblastoma; Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Solid tumours | Details |
| Necitumumab | LY-3012211; IMC-11F8 | Approved | Eli Lilly And Company | Portrazza | Japan | Carcinoma, Non-Small-Cell Lung | Nippon Kayaku Co Ltd | 2015-11-24 | Solid tumours; Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Afatinib Dimaleate | BIBW-2992; BIBW-2992-MA2 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Gilotrif, Tomtovok, 吉泰瑞, Tovok, Giotrif | Mainland China | Carcinoma, Non-Small-Cell Lung | Boehringer Ingelheim International Gmbh | 2013-07-12 | Lymphoma; Prostatic Neoplasms; Brain Neoplasms; Urethral Neoplasms; Colorectal Neoplasms; Urologic Neoplasms; Ureteral Neoplasms; Carcinoma, Squamous Cell; Lung Neoplasms; Breast Neoplasms; Uterine Neoplasms; Glioma; Esophageal Squamous Cell Carcinoma; Gallbladder Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Hematologic Neoplasms; Liver Diseases; Urinary Bladder Neoplasms; Chordoma; Multiple Myeloma; Neuroectodermal Tumors; Glioblastoma; Neoplasms; Neoplasms, Squamous Cell; Renal Insufficiency; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Rhabdomyosarcoma; Head and Neck Neoplasms; Solid tumours | Details |
| Erlotinib Hydrochloride | CP-358774-01; RO-0508231; NSC-718781; CP-358774; RG-1415; OSI-774; R-1415 | Approved | Genentech Inc | Tarceva, 特罗凯 | Japan | Carcinoma, Non-Small-Cell Lung | Chugai Pharmaceutical Co Ltd | 2004-11-18 | Urologic Neoplasms; Brain metastases; Esophageal adenocarcinoma; Lung Neoplasms; Lip Neoplasms; Mouth Neoplasms; Carcinoma, Mucoepidermoid; Bile Duct Neoplasms; Adenocarcinoma, Bronchiolo-Alveolar; Oropharyngeal Neoplasms; Neuroectodermal Tumors, Primitive; Ureteral Neoplasms; Astrocytoma; Glioma; Gliosarcoma; Colorectal Neoplasms; Psoriasis; Osteosarcoma; Sarcoma; Cholangiocarcinoma; Metaplasia; Urethral Neoplasms; Neuroblastoma; Brain Neoplasms; Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Endometrioid; Melanoma; Neoplasms, Germ Cell and Embryonal; Breast Neoplasms, Male; Meningioma; Precancerous Conditions; Neoplasm Metastasis; Uterine Cervical Neoplasms; Tongue Neoplasms; Prostatic Neoplasms; Laryngeal Neoplasms; Adenomatous Polyps; Lymphoma; Gallbladder Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Squamous Cell; Adenocarcinoma, Mucinous; Paranasal Sinus Neoplasms; Neoplasms, Unknown Primary; Endometrial Neoplasms; Fallopian Tube Neop | Details |
| Cetuximab | IMC-C255; BMS-564717; EMD-271786; C-255; GT-MAB-5.2; ch-225; LY-2939777; NSC-714692 | Approved | Bristol-Myers Squibb Company, Eli Lilly And Company, Merck Serono | 爱必妥, Erbitux | Mainland China | Squamous Cell Carcinoma of Head and Neck | Merck Serono Co Ltd | 2004-02-12 | Endometrial Neoplasms; Oropharyngeal Neoplasms; Peritoneal Neoplasms; Neuralgia; Fallopian Tube Neoplasms; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Laryngeal Neoplasms; Carcinoma, Squamous Cell; Colorectal Neoplasms; Adenocarcinoma, Mucinous; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Precancerous Conditions; Complex Regional Pain Syndromes; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Colonic Neoplasms; Head and Neck Neoplasms; Neoplastic Cells, Circulating; Liver Neoplasms; Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Pain; Squamous Cell Carcinoma of Head and Neck; Ovarian Neoplasms; Neoplasms; Pancreatic Neoplasms; Hypopharyngeal Neoplasms; Neoplasms, Squamous Cell; Carcinoma, Adenoid Cystic; Nasopharyngeal Carcinoma; Sarcoma | Details |
| Lapatinib Ditosylate Hydrate | GW-572016; GW-572016F; GW-2016 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | Tykerb, Tyverb, 泰立沙, Tykerb/Tyverb | Mainland China | Breast Neoplasms | Glaxosmithkline Ag | 2007-03-13 | Neoplasms, Gonadal Tissue; Carcinoma, Acinar Cell; Carcinoma, Mucoepidermoid; Colorectal Neoplasms; Astrocytoma; Gliosarcoma; Bile Duct Neoplasms; Carcinoma, Squamous Cell; Lymphoma; Glioma; Brain metastases; Lung Neoplasms; Endometrial Neoplasms; Brain Neoplasms; Laryngeal Neoplasms; Gallbladder Neoplasms; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular; Neoplasm Metastasis; Tongue Neoplasms; Melanoma; Breast Neoplasms, Male; Carcinoma, Non-Small-Cell Lung; Neuroma, Acoustic; Pancreatic Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms; Ependymoma; Liver Neoplasms; Medulloblastoma; Stomach Neoplasms; Esophageal Neoplasms; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma; Carcinoma, Ovarian Epithelial; Solid tumours; Carcinoma, Verrucous; Glioblastoma; Salivary Gland Neoplasms; Neoplasms; Neurofibromatosis 2; Urinary Bladder Neoplasms; Carcinoma, Adenoid Cystic; Central Nervous System Neoplasms; Oligodendroglioma; Breast Neoplasms; Prost | Details |
| Gefitinib | ZD-1839 | Approved | Astrazeneca Pharmaceutical Co Ltd | 易瑞沙, Iressa | EU | Carcinoma, Non-Small-Cell Lung | Astrazeneca Ab | 2002-07-05 | Fallopian Tube Neoplasms; Gastrinoma; Prostatic Neoplasms; Brain Neoplasms; Urethral Neoplasms; Breast Neoplasms; Astrocytoma; Gliosarcoma; Colorectal Neoplasms; Adenocarcinoma, Bronchiolo-Alveolar; Carcinoma, Mucoepidermoid; Lung Neoplasms; Neoplasms, Neuroepithelial; Glioma; Carcinoma, Adenosquamous; Endometrial Neoplasms; Carcinoma, Squamous Cell; Leukemia, Myeloid, Acute; Brain metastases; Carcinoma, Non-Small-Cell Lung; Neoplasms, Germ Cell and Embryonal; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Somatostatinoma; Neoplasm Metastasis; Adenocarcinoma; Glioblastoma; Liver Neoplasms; Solid tumours; Kidney Neoplasms; Ovarian Neoplasms; Carcinoma, Islet Cell; Vipoma; Insulinoma; Carcinoid Tumor; Abdominal Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Carcinoma; Stomach Neoplasms; Head and Neck Neoplasms; Skin Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Adenocarcinoma of Lung; Small Cell Lung Carcinoma; Salivary Gland Neoplasms; | Details |
| Osimertinib Mesylate | AZD-9291; RDL94R2A16; AZD-9291 Mesylate; AZD9291 | Approved | Astrazeneca Pharmaceutical Co Ltd | 泰瑞沙, Tagrisso | Mainland China | Carcinoma, Non-Small-Cell Lung | Astrazeneca Ab | 2015-11-13 | Uterine Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Meningeal Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Lung Neoplasms; Brain metastases; Esophageal adenocarcinoma; Cholangiocarcinoma; Lymphoma; Glioma; Endometrial Neoplasms; Thyroid Neoplasms; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Adenocarcinoma of Lung; Liver Neoplasms; Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Rectal Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Solid tumours; Carcinoma, Ovarian Epithelial; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Skin Neoplasms; Colonic Neoplasms; Glioblastoma; Multiple Myeloma; Urinary Bladder Neoplasms | Details |
| Brigatinib | AP-26113 | Approved | Ariad, Takeda Pharmaceutical Co Ltd | Alunbrig | Mainland China | Carcinoma, Non-Small-Cell Lung | Takeda (China) International Trading Co Ltd | 2017-04-28 | Solid tumours; Ependymoma; Carcinoma; Neoplasms; Neurofibromatosis 2; Myofibroma; Lymphoma, Large-Cell, Anaplastic; Granuloma, Plasma Cell; Lung Neoplasms; Brain metastases; Carcinoma, Non-Small-Cell Lung; Sarcoma, Kaposi; Meningioma; Neurilemmoma; Neuroma, Acoustic | Details |
| Pyrotinib Maleate | HTI-1001; SHR-1258; BLTN | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾瑞妮 | Mainland China | Breast Neoplasms | Jiangsu Hengrui Medicine Co Ltd | 2018-08-12 | Solid tumours; Biliary Tract Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Adenocarcinoma of Lung; Neoplasms; Breast Neoplasms; Bile Duct Neoplasms; Metastatic breast cancer; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms | Details |
| Amivantamab | JNJ-372; JNJ-61186372 | Approved | Janssen Global Services Llc, Genmab A/S | RYBREVANT | Canada | Carcinoma, Non-Small-Cell Lung | Janssen Inc | 2021-05-21 | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Panitumumab | ABX-0303; E7.6.3; AMG-954; ABX-EGF; ABX-10221 | Approved | Amgen Inc | Vectibix | Japan | Colorectal Neoplasms | Takeda | 2006-09-27 | Salivary Gland Neoplasms; Gastrointestinal Neoplasms; Exanthema; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Glioma; Carcinoma, Squamous Cell; Lung Neoplasms; Adenoma, Pleomorphic; Colorectal Neoplasms; Brain Neoplasms; Prostatic Neoplasms; Kidney Neoplasms; Malignant Carcinoid Syndrome; Pancreatic Neoplasms; Colonic Neoplasms; Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma; Rectal Neoplasms; Esophageal Neoplasms; Head and Neck Neoplasms; Solid tumours | Details |
| Neratinib Maleate | CAN-030; HKI-272; PF-0528767; WAY-179272; PB-272 | Approved | Pfizer Pharmaceuticals Ltd (China) | Nerlynx, 贺俪安 | Mainland China | Breast Neoplasms | Excella Gmbh & Co Kg | 2017-07-17 | Solid tumours; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Lung Neoplasms; Metastatic breast cancer; Brain metastases; Carcinoma, Non-Small-Cell Lung | Details |
| Lazertinib | YH-25448; JNJ-73841937; JNJ-1937; GNS-1480 | Approved | Oscotec Inc, Janssen Biotech Inc, Yuhan Corp | Leclaza | South Korea | Carcinoma, Non-Small-Cell Lung | Yuhan Corp | 2021-01-18 | Hepatic Insufficiency; Carcinoma, Non-Small-Cell Lung | Details |
| Nimotuzumab | OSAG-10; KI-0502; KI-0501; DE-766; YMB-1000; OSAG-101; h-R3; TheraCIMh-R3 | Approved | Center Of Molecular Immunology, Cimym Biosciences | Theraloc, BIOMAb-EGFR, TheraCIM, 泰欣生, CIMAher | Mainland China | Nasopharyngeal Neoplasms | Biotech Pharmaceuticals Co Ltd | 2006-07-21 | Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Anus Neoplasms; Neoplasms; Nasopharyngeal Neoplasms; Pancreatic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Nasopharyngeal Diseases; Nasopharyngeal Carcinoma; Esophageal Squamous Cell Carcinoma; Glioma; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Esophageal Diseases | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Humanized anti-EGFR monoclonal antibody (Hualan Biological Engineering/Henan shengming biotechnology) | Phase 2 Clinical | Henan Shengming Biotechnology Research Institute Co Ltd, Hualan Genetic Engineering Co Ltd | Colorectal Neoplasms | Details | |
| WTS-004 | WTS-004 | Phase 1 Clinical | Hangzhou Wutong Tree Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| JRF-103 | JRF103; JRF-103 | Phase 2 Clinical | Shenzhen Jinrui Foundation Biotechnology Co Ltd | Solid tumours | Details |
| Anti-CTLA-4/PD-1 expressing EGFR-CAR-T | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Solid tumours | Details | |
| EGFR IL12 CART | Phase 1 Clinical | Shenzhen Prekin Biopharmaceutical Co Ltd | Colorectal Neoplasms | Details | |
| Losatuxizumab vedotin | ABBV221; ABBV-221 | Phase 1 Clinical | Abbvie Inc | Neoplasms | Details |
| Cetuximab biosimilar (Enzene Biosciences) | Clinical | Enzene Biosciences Ltd | Carcinoma, Squamous Cell | Details | |
| PB-357 | PB-357 | Phase 1 Clinical | Pfizer Inc | Neoplasms | Details |
| SGT-210 | SGT-210 | Phase 1 Clinical | Sol Gel Technologies Pte Ltd | Keratosis | Details |
| Cetuximab I-131 (Pacific Meinuoke) | Clinical | Jiangsu Pacific-Meinuoke Bio-Pharmaceutical Co Ltd | Colorectal Neoplasms | Details | |
| KSP-QRH-E3-IRDye800 | Phase 1 Clinical | University Of Michigan | Cholangiocarcinoma | Details | |
| Betatinib | TL-512 | Phase 1 Clinical | Aspedia Llc, Suzhou Teligene Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Cetuximab biobetter (Mabtech/Sorrento) | STI-001 | Phase 3 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd, Shanghai Biomabs Pharmaceuticals Co Ltd | Colorectal Neoplasms | Details |
| EGFRvIII-CAR | Phase 1 Clinical | Duke University Medical Center | Glioblastoma | Details | |
| Recombinant chimeric anti-EGFR monoclonal antibody (Qilu Pharmaceutical) | QL-1105 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours | Details |
| Cetuximab biosimilar (AlphaMab) | KN-005 | Phase 3 Clinical | Head and Neck Neoplasms; Anus Neoplasms; Urinary Bladder Neoplasms; Colorectal Neoplasms | Details | |
| HER-1 vaccine (Center of Molecular Immunology) | HER1-ECD; HER1-VSSP; HER-1-ECD-VSSP | Phase 2 Clinical | Center Of Molecular Immunology | Neoplasms | Details |
| Durvalumab/Gefitinib | Phase 2 Clinical | Medimmune | Carcinoma, Non-Small-Cell Lung | Details | |
| Anti-EGFR CAR T-cell therapy (Seattle Children's Hospital) | EGFR-806 | Phase 1 Clinical | Seattle Children'S Hospital | Central Nervous System Neoplasms | Details |
| Nimotuzumab biosimilar (IBC Generium) | Phase 3 Clinical | International Biotechnology Center Generium Llc | Head and Neck Neoplasms | Details | |
| BC-3448 | BC-3448; BC3448 | Phase 1 Clinical | Wuxi Zhikang Hongyi Biological Technology Co Ltd | Solid tumours | Details |
| Recombinant anti-InE monoclonal antibody | CmAb-(IL10)2 | Phase 1 Clinical | Dingfu Biotarget Co Ltd | Solid tumours | Details |
| BEBT-109 | BEBT-109 | Phase 2 Clinical | Guangzhou BeBetter Medicine Technology Co | Carcinoma, Non-Small-Cell Lung | Details |
| SMET-12 | SMET-12 | Phase 1 Clinical | Zhejiang Shimai Pharmaceutical Co Ltd | Solid tumours | Details |
| Sirotinib Maleate | XZP-5491 | Phase 1 Clinical | Shandong Xuanzhu Pharmaceutical Technology Co Ltd | Stomach Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
| Puvitinib | Phase 1 Clinical | Suzhou Teligene Ltd | Solid tumours | Details | |
| KY-1701 | KY-1701 | Phase 1 Clinical | Jiangsu Kanion Pharmaceutical Co Ltd | Breast Neoplasms | Details |
| Epertinib | S-222611 | Phase 2 Clinical | Shionogi & Co Ltd | Neoplasms | Details |
| WSD-0922 | WSD0922; WSD-0922 | Phase 1 Clinical | Vision Biological Tech (Hefei) Co Ltd | Glioblastoma; Glioma | Details |
| Mefatinib | Phase 3 Clinical | Suzhou Maitai Bio-Technology Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details | |
| CMAB-017 | CMAB-017 | Phase 1 Clinical | Taizhou Mabtech Pharmaceutical Co Ltd | Solid tumours | Details |
| Anti-CD3/anti-EGFR -activated T cells (Barbara Ann Karmanos Cancer Institute) | Barbara Ann Karmanos Cancer Institute, University Of Virginia | Details | |||
| ZZ-06 | ZZ-06 | Phase 1 Clinical | Changchun Intellicrown Pharmaceutical Co Ltd | Solid tumours | Details |
| ABBV-637 | ABBV-637 | Abbvie Inc | Details | ||
| FWD1509 MsOH | FWD1509 MsOH | Phase 1 Clinical | Shenzhen Forward Pharmaceuticals Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| [111In] Panitumumab | Stanford University | Details | |||
| BB-1705 | BB-1705 | Phase 1 Clinical | Baili Sikang Biomedicine (Hangzhou) Co Ltd | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| EGFR/B7H3 CAR-T | EGFR/B7H3 CAR-T | Second Affiliated Hospital Of Guangzhou Medical University | Details | ||
| JMT101 | JMT-101 | Phase 3 Clinical | Shanghai Jinmante Biological Technology Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| MP-412 | AV-412; MP-412 | Mitsubishi Tanabe Pharma | Details | ||
| Recombinant anti-EGFR chimeric monoclonal antibody (Shanghai CP Guojian) | CPGJ-602; 602; CPGJ602; CPGJ 602 | Phase 2 Clinical | Shanghai Cp Guojian Pharmaceutical Co Ltd | Colorectal Neoplasms | Details |
| Cimaglermin alfa | CGF-2 | Ludwig Institute For Cancer Research | Details | ||
| TAK-285 | TAK-285 | Takeda | Details | ||
| Immunomodulatory progenitor cell therapy (Celixir) | Phase 3 Clinical | Celixir | Cardiomyopathies | Details | |
| BB-101 (Blue Blood Biotech/National Cheng Kung University) | BB-101 | National Cheng Kung University, Blue Blood Biotech Corp | Details | ||
| Cetuximab biosimilar (R-Pharm) | RPH-002 | Phase 3 Clinical | R-Pharm | Head and Neck Neoplasms | Details |
| Nimotuzumab biosimilar (El Kendi Pharmaceuticals Manufacturing) | El Kendi Pharmaceuticals | Details | |||
| TTI-1612 | TTI-1612 | Details | |||
| TQB-3456 | TQB-3456 | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd, Lianyungang Runzhong Pharmaceutical Co Ltd, Centaurus Biopharma Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| PF-06459988 | PF-6459988; PF-06459988 | Pfizer Pharmaceuticals Ltd (China) | Details | ||
| EMD 55900 | EMD-55900 | Merck Serono | Details | ||
| Mavelertinib | PF-7775; PFE-X775; PF6747775; PF-06747775 | Pfizer Pharmaceuticals Ltd (China) | Details | ||
| MP-0274 | DARPin-41; SPA-28; CME-114; CME-115; CME-118; CME-119; MP-0274 | Molecular Partners Ag | Details | ||
| Olafertinib | CK-101; RX-518; CS-2481; EGFR-IN-3 | Phase 3 Clinical | Suzhou Neupharma Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Sutetinib Maleate | Phase 2 Clinical | Jiangsu Maidu Pharmaceutical R & D Co Ltd, Jiangsu Suzhong Pharma Group Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details | |
| BPI-15086 | BPI-15000; BPI-15086 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Nazartinib | EGF-816; EGFRmut-TKI EGF816 | Phase 2 Clinical | Novartis Pharma Ag | Bronchial Neoplasms; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| GC-1118A | GC-1118; GC-1118A | Phase 2 Clinical | Korean Green Cross Corp | Solid tumours; Stomach Neoplasms; Colorectal Neoplasms; Neoplasm Metastasis | Details |
| IAE-0972 | IAE-0972 | Phase 2 Clinical | Shenghe (China) Biopharmaceutical Co Ltd | Solid tumours | Details |
| Recombinant anti-EGFR chimeric antibody (Harbin Pharmaceutical) | Phase 1 Clinical | Harbin Pharmaceutical Group Holding Co Ltd | Colorectal Neoplasms | Details | |
| Cetuximab biosimilar (Humanwell Healthcare) | Phase 1 Clinical | Humanwell Healthcare (Group) Co Ltd | Colorectal Neoplasms | Details | |
| Neptinib Di-P-methylbenzenesulfonate | Phase 1 Clinical | Shenzhen Neptunus Pharmaceutical Research Institute Co Ltd | Stomach Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| LY-01010 | LY-01010 | Phase 1 Clinical | Luye Pharma Group Ltd | Breast Neoplasms | Details |
| HS-10376 | Phase 2 Clinical | Jiangsu Hansoh Pharmaceutical Group Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details | |
| Selatinib Ditosilate | QLNC-120 | Phase 2 Clinical | Qilu Antibiotics (Linyi) Pharmaceutical Co Ltd, Qilu Pharmaceutical Co Ltd | Breast Neoplasms | Details |
| Allitinib Tosylate | AST-6; ALS-1306; AST-1306 | Phase 2 Clinical | Shanghai Allist Pharmaceutical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AMX-3009 Maleate | AMX-3009; AMX3009马来酸 | Phase 1 Clinical | Anrun Medicine Technology (Suzhou) Co Ltd | Solid tumours; Stomach Neoplasms; Pancreatic Neoplasms; Breast Neoplasms | Details |
| EG-007 | EG-007 | Phase 3 Clinical | Evergreen Therapeutics Inc | Endometrial Neoplasms | Details |
| CKD-702 | Phase 1 Clinical | Chong Kun Dang Pharmaceutical Corp | Carcinoma, Non-Small-Cell Lung | Details | |
| NIP-142 | NIP-142 | Phase 1 Clinical | Carcinoma, Non-Small-Cell Lung | Details | |
| Recombinant chimeric anti-EGFR monoclonal antibody (Zhejiang Xinwei Shengke Biotechnology) | Phase 1 Clinical | Zhejiang Xinweishengke Biotechnology Co Ltd | Esophageal Neoplasms | Details | |
| ORIC-114 | ORIC-114 | Phase 1 Clinical | Voronoi | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| Double-deleted Vaccinia Virus Plus CD/ SMR | JX-929; vvDD-CDSR | Phase 1 Clinical | Sillajen Inc | Liver Neoplasms; Squamous Cell Carcinoma of Head and Neck; Pancreatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Melanoma | Details |
| Simotinib Hydrochloride | AL-6802; SIM-6802 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Advenchen Laboratories Llc | Carcinoma, Non-Small-Cell Lung | Details |
| ZN-e4 | ZN-e4; KP-673 | Phase 2 Clinical | Zentalis Pharmaceuticals LLC, Zeno Pharma | Carcinoma, Non-Small-Cell Lung | Details |
| BBT-176 | BBT-176 | Phase 2 Clinical | Bridge Biotherapeutics Inc | Carcinoma, Non-Small-Cell Lung | Details |
| Anti-EGFR-IL-dox (Swiss Group for Clinical Cancer Research) | Phase 2 Clinical | Schweizerische Arbeitsgemeinschaft Für Klinische Krebsforschung | Breast Neoplasms | Details | |
| Hemay-020 | Hemay-020 | Phase 1 Clinical | Tianjin Hemay Pharmaceutical Co Ltd, Hainan General Sanyang Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| Anti-EGFR-bispecific antibody armed activated T-cell therapy (Memorial Sloan Kettering Cancer Center) | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Pancreatic Neoplasms | Details | |
| C225-ILS-DOX | C225-ILs-dox | Phase 2 Clinical | Universitaetsspitals Basel | Breast Neoplasms | Details |
| D2C7-based immunotoxins (Duke University) | D2C7-IT; scds-D2C7 -PE38KDEL; D2C7-(scdsFv)-PE38 KDEL | Phase 1 Clinical | Istari Oncology Inc, Duke University | Glioma | Details |
| ABY-029 | ABY-029 | Phase 1 Clinical | Dartmouth College, Affibody Ab, Li-Cor Bioscience | Head and Neck Neoplasms; Sarcoma; Glioma | Details |
| Autologous EGFR-CAR T cells (Bio-gene) | Phase 1 Clinical | Sun Yat-Sen University, Guangzhou Bio-Gene Technology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | |
| M-1231 | M-1231 | Phase 1 Clinical | Emd Serono Research & Development Institute Inc, Merck Serono | Solid tumours; Esophageal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Anti-EGFR CAR-T cell therapy (Beijing Pregene) | Phase 2 Clinical | Shenzhen Prekin Biopharmaceutical Co Ltd | Colorectal Neoplasms | Details | |
| AP-L1898 | JS111; AP-L1898; WJ-002 | Phase 2 Clinical | Suzhou Junjing Biomedical Technology Co Ltd, Wigen Biomedicine technology (Shanghai) Co Ltd, Shanghai Junshi Biosciences Co Ltd | Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| TAK-186 | EGFR x CD3 COBRA; MVC-101; TAK-186 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| E-EDV-D682 | PNU-15982; E-EDV-D682; EGFR-EDV-PNU-15982 | Phase 2 Clinical | Engeneic Ltd | Pancreatic Neoplasms | Details |
| Antibody-drug nanocell conjugates (EnGeneIC) | EGFR-EDV-RRM1; EGFR-EDV-PLK; EGFR-EDV-Dox | Phase 1 Clinical | Engeneic Ltd | Glioblastoma | Details |
| H-002 | H-002 | Phase 2 Clinical | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| DBPR-112 | DBPR-112; ABT-101 | Phase 2 Clinical | National Health Research Institutes | Head and Neck Neoplasms; Solid tumours; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| YK-029A | YK-029A | Phase 1 Clinical | Hainan Yuekang Biomedicine Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| ZSP-0391 | ZSP-0391 | Phase 1 Clinical | Wuxi Apptec Co Ltd, Guangdong Zhongsheng Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| SYN-004 (Synermore Biologics) | LR-004; SYN-004 | Phase 1 Clinical | Lonn Ryonn Pharma Ltd, Synermore Biologics (Suzhou) Co Ltd | Solid tumours; Carcinoma; Lymphoma, Large B-Cell, Diffuse; Colorectal Neoplasms | Details |
| PLB-1004 | PLB-1004 | Phase 1 Clinical | Beijing Anshi Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| REGN-7075 | REGN-7075 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Solid tumours; Neoplasms | Details |
| BLU-451 | BLU-451 | Phase 2 Clinical | Blueprint Medicines Corp | Carcinoma, Bronchogenic; Bronchial Neoplasms; Carcinoma; Respiratory Tract Diseases; Neoplasms; Lung Diseases; Respiratory Tract Neoplasms; Neoplasms, Nerve Tissue; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| GB-263 | GB-263; GB-263T | Phase 2 Clinical | Genor Biopharma Co Ltd | Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Demupitamab | SCT-200 | Phase 2 Clinical | SinoCelltech Ltd | Solid tumours; Head and Neck Neoplasms; Biliary Tract Neoplasms; Ovarian Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma; Pancreatic Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Esophageal Squamous Cell Carcinoma; Gallbladder Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Sapitinib | AZD-8931 | Phase 2 Clinical | Astrazeneca Pharmaceutical Co Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| TQ-B3395 | TQ-B3395; TQ-B-3395 | Phase 1 Clinical | Centaurus Biopharma Co Ltd, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Neoplasms; Breast Neoplasms | Details |
| Cetuximab biosimilar (Shanghai Zhangjiang Biotechnology) | STI-001; CMAB-009 | Phase 3 Clinical | Shanghai Zhangjiang Biotechnology Co Ltd | Neoplasms; Colorectal Neoplasms | Details |
| TQB3804 | TQB-3804 | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Solid tumours; Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Cetuximab biosimilar (Guilin Sanjin) | CDP-1; BC-001 | Phase 1 Clinical | Dragonboat Biopharmaceutical, Guilin Sanjin Pharmaceutical Co Ltd | Solid tumours; Neoplasms; Colorectal Neoplasms | Details |
| CART-EGFR-IL13Ra2 | CART-EGFR-IL13Ra2; CAR-T-EGFR-IL-13-Ra-2 | Phase 1 Clinical | University Of Pennsylvania | Glioblastoma | Details |
| Futuximab/Modotuximab | 992-and-1024; Sym-004; S95026; S-95026 | Phase 3 Clinical | Symphogen A/S | Solid tumours; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Glioma | Details |
| QLH-11811 | QLH-11811; QLH11811 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| BNA-035 | BNA-035 | Phase 1 Clinical | Binacea Pharma Inc | Solid tumours | Details |
| BLU-701 | BLU 701; BLU-701 | Phase 2 Clinical | Blueprint Medicines Corp | Carcinoma, Bronchogenic; Carcinoma; Thoracic Neoplasms; Bronchial Neoplasms; Respiratory Tract Diseases; Neoplasms; Lung Diseases; Respiratory Tract Neoplasms; Neoplasms, Nerve Tissue; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| NRC-2694 | NRC-2694; NRC-2694A; NRC-2694-A | Phase 2 Clinical | Natco Pharma | Carcinoma; Squamous Cell Carcinoma of Head and Neck | Details |
| Bafisontamab | FIT-013a; EMB-01 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Liver Neoplasms; Biliary Tract Neoplasms; Carcinoid Tumor; Stomach Neoplasms; Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular | Details |
| Cetuximab biosimilar (AMPO Biotechnology) | Phase 3 Clinical | Ampo Biotechnology Inc | Colorectal Neoplasms | Details | |
| MCLA-129 | MCLA-129 | Phase 2 Clinical | Merus Nv | Solid tumours; Head and Neck Neoplasms; Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AFM-24 | AFM-24 | Phase 2 Clinical | Affimed | Solid tumours | Details |
| [89Zr]Panitumumab (University of Alabama at Birmingham) | Phase 1 Clinical | University Of Alabama At Birmingham | Squamous Cell Carcinoma of Head and Neck; Colonic Neoplasms; Pancreatic Neoplasms | Details | |
| Cetuximab biosimilar (Kelun Group) | KL-A140; KLA140; A-140; KL-140; KLA-140 | Phase 3 Clinical | Sichuan Kelun Pharmaceutical Co Ltd | Rectal Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Adenocarcinoma | Details |
| BPI-361175 | BPI-361175 | Phase 2 Clinical | Betta Pharmaceuticals Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| TAS-6417 | CLN-081; TAS-6417 | Phase 2 Clinical | Taiho Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Tarloxotinib Bromide | PR-610; TH-4000; SN-33999 | Phase 2 Clinical | Threshold | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| Petosemtamab | MCLA-158 | Phase 1 Clinical | Merus Nv | Neoplasms; Colorectal Neoplasms | Details |
| Pimurutamab | HLX-07 | Phase 2 Clinical | Shanghai Henlius Biotech Co Ltd | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Adenosquamous; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Lung Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| HS-10375 | HS-10375 | Phase 2 Clinical | Jiangsu Hansoh Pharmaceutical Group Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| BDTX-1535 | BDTX-1535 | Phase 1 Clinical | Black Diamond Therapeutics Inc | Glioblastoma; Carcinoma, Non-Small-Cell Lung | Details |
| MRG003 | MRG-003 | Phase 2 Clinical | Shanghai Miracogen Inc | Head and Neck Neoplasms; Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Bile Duct Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| DWP-708 | DWP-708 | Phase 2 Clinical | Daewoong Pharmaceutical Co Ltd | Acneiform Eruptions | Details |
| TAVO-412 | TAVO-412 | Phase 1 Clinical | Tavotek Biotherapeutics (Hong Kong) Ltd | Neoplasms | Details |
| Tesevatinib | XL-647; KD-020; KD-019; EXEL-7647 | Phase 2 Clinical | Exelixis Inc | Neoplasms; Glioblastoma; Breast Neoplasms; Brain Neoplasms; Polycystic Kidney, Autosomal Dominant; Brain metastases; Polycystic Kidney, Autosomal Recessive; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| WJ-13404 | WJ-13404; JS-113; WJ-004 | Phase 1 Clinical | Wigen Biomedicine technology (Shanghai) Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant human anti-EGFR mAb (Serum Biotechnology) | SY-101 | Phase 2 Clinical | Shanghai Serum Bio-Technology Co Ltd, Institute of Bioengineering Academy of Military Medical Sciences Chinese People's Liberation Army | Solid tumours; Colorectal Neoplasms | Details |
| Panitumumab-IRDye800CW (Stanford University) | Phase 2 Clinical | Stanford University | Head and Neck Neoplasms; Brain Neoplasms | Details | |
| BAY-2927088 | BAY-2927088; BAY2927088 | Phase 1 Clinical | Bayer Healthcare Company Ltd, Bayer AG | Carcinoma, Non-Small-Cell Lung | Details |
| Larotinib Mesylate | Z-650 | Phase 3 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Pancreatic Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| Sunvozertinib | DZD-9008 | Phase 2 Clinical | Dizal (Jiangsu) Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung | Details |
| SKLB-1028 | SKLB-1028 | Phase 3 Clinical | Sichuan University, CSPC Pharmaceutical Group Ltd | Solid tumours; Leukemia, Promyelocytic, Acute; Leukemia, Myeloid; Leukemia, Myeloid, Acute | Details |
| QL1203 | QL-1203 | Phase 3 Clinical | Qilu Pharmaceutical Co Ltd | Colorectal Neoplasms | Details |
| HA121-28 | HA121-28; SYHA121-28 | Phase 3 Clinical | CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co Ltd | Biliary Tract Neoplasms; Solid tumours; Esophageal Neoplasms; Neoplasms; Medullary thyroid cancer (MTC); Bile Duct Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| NX-019 | NX-019 | Phase 1 Clinical | Nalo Therapeutics Inc | Neoplasms | Details |
| TAS-2940 | TAS-2940 | Phase 1 Clinical | Taiho Oncology Inc | Solid tumours; Glioblastoma; Breast Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BL-B01D1 | Phase 1 Clinical | Sichuan Baili Pharmaceutical Co Ltd | Neoplasms, Fibroepithelial; Solid tumours; Neoplasms; Digestive System Neoplasms; Breast Neoplasms; Urologic Neoplasms; Gastrointestinal Neoplasms | Details | |
| Zorifertinib | AZD-3759 | Phase 3 Clinical | Astrazeneca Plc | Brain metastases; Carcinoma, Non-Small-Cell Lung | Details |
| FCN-411 | FCN-411 | Phase 2 Clinical | Shanghai Fosun Pharmaceutical (Group) Co Ltd | Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CX-904 | CX-904 | Phase 1 Clinical | Amgen Inc, Cytomx Therapeutics Inc | Solid tumours; Neoplasms | Details |
| Epitinib Succinate | HMPL-813 | Phase 1 Clinical | Solid tumours; Neoplasms; Glioblastoma; Carcinoma, Non-Small-Cell Lung | Details | |
| Pirotinib Hydrochloride | KBP-5209 | Phase 2 Clinical | Shandong Xuanzhu Pharmaceutical Technology Co Ltd | Solid tumours; Neoplasms; Breast Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Varlitinib Ditosylate | QBT-01; ARRY-543; SPS-4370; ASLAN-001; ARRY-334543 | Phase 2 Clinical | Array Biopharma | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Bile Duct Neoplasms | Details |
| Antroquinonol | Phase 2 Clinical | Golden Biotechnology Corporation Ltd | Pancreatic Neoplasms; Coronavirus Disease 2019 (COVID-19); Hepatitis B; Leukemia, Myeloid, Acute; Carcinoma, Non-Small-Cell Lung; Hyperlipidemias; Dermatitis, Atopic | Details | |
| ES-072 | ES-072 | Phase 1 Clinical | Zhejiang Bosheng Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Depatuxizumab mafodotin | ABT-414/806; ABT-414 | Phase 3 Clinical | Abbvie Inc | Glioblastoma; Gliosarcoma; Glioma; Carcinoma, Squamous Cell | Details |
| HLX-35 | HLX-35 | Phase 1 Clinical | Shanghai Henlius Biologics Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| Serclutamab talirine | ABBV-321 | Phase 1 Clinical | Abbvie Inc | Neoplasms | Details |
| Izalontamab | SI-B001; SI-1X6.4 | Phase 3 Clinical | Sichuan Baili Pharmaceutical Co Ltd | Head and Neck Neoplasms; Solid tumours; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Neoplasms, Glandular and Epithelial; Triple Negative Breast Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| Cetuximab biosimilar (Shanghai Henlius Biotech) | HLX-05; JZB-29; JZB-28 | Phase 1 Clinical | Shanghai Henlius Biotech Co Ltd | Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms | Details |
| JNJ-26483327 | JNJ-26483327; MTKi-327; BGB-102 | Phase 2 Clinical | Johnson & Johnson | Solid tumours; Neoplasms; Macular Degeneration | Details |
This web search service is supported by Google Inc.
