
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































> Insights > Harnessing Growth Factors and Cytokines for Enhanced Dendritic Cell (DC) proliferation and differentiation Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) in the human body, often referred to as the “sentinels” of the immune system. They play a central role in initiating, regulating, and sustaining innate and adaptive immune responses, interacting with various immune cells such as T cells, B cells, etc., to enhance the effectiveness of immune responses. By leveraging the unique physiological advantages of DCs, DC vaccines have emerged as a highly pursued topic in cancer immunotherapy. These vaccines can be broadly categorized into three types based on preparation strategies:
1. Early-stage DC vaccines which utilize antigens in the form of proteins or long peptides along with adjuvants to promote DC maturation.
2. In vivo approach DC vaccines.
3. Ex vivo DC vaccines load DCs with antigens ex vivo before reintroduction into the body. This method, also termed DC cell therapy, has shown promise in the prevention/treatment of various infectious diseases and malignancies.
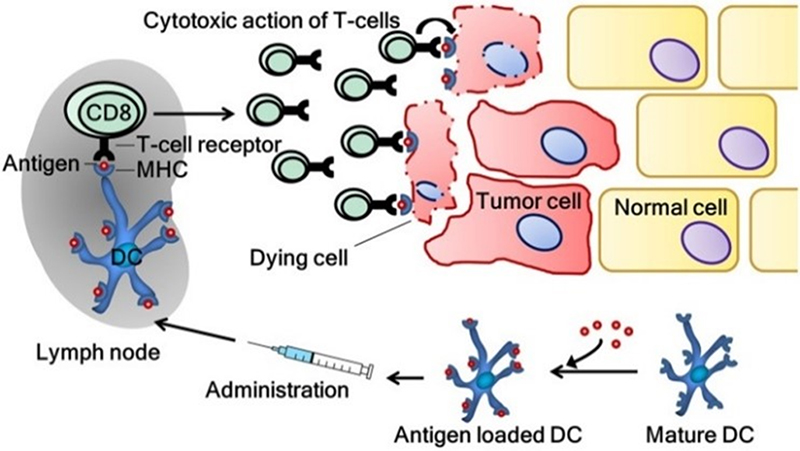
The induction of a tumor-specific immune response by dendritic cell vaccination. Frontiers in immunology, 9, 2265.
In this approach, either monocytes are first isolated from the patient's blood or CD34+ precursor cells are sourced from the bone marrow. Cells are then subjected to stimulation and differentiation using various cytokines, resulting in the generation of immature dendritic cells (DCs). These immature DCs are loaded with antigens, ranging from nucleic acids to peptides, proteins, cells, or nanoparticles, through methods such as co-culture, cell fusion, or transfection. Further stimulation is applied to induce maturation, leading to the development of mature DCs ready for reintroduction into the patient's body. Once reintroduced, these matured DCs process the antigens and present them to the immune system, initiating or enhancing the body's immune response against target antigens.
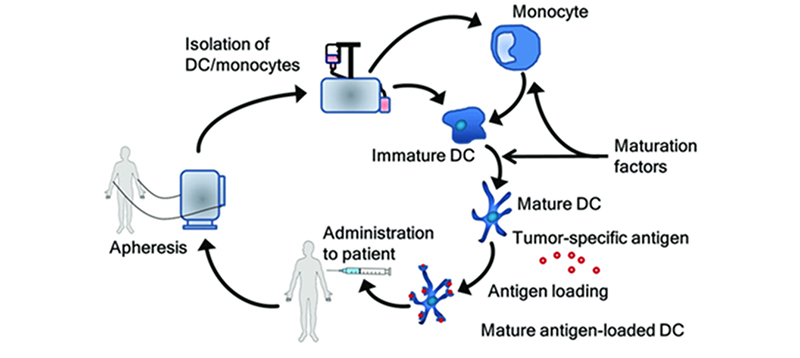
The process of generating dendritic cell vaccines. Frontiers in immunology, 9, 2265.
Ex vivo culturing of DCs is the most time-consuming step throughout the manufacturing of DC vaccines, requiring stringent procedures and precisely controlled conditions. The differentiation and maturity of DCs, which are required for DC vaccine production, are particularly important. During clinical production, the differentiation and maturation of DCs occurs within a combination of cytokine-rich culture environments. Among these growth factors, GM-CSF, IL-4, and TNF-α are considered the three main growth factors for DC maturation and differentiation. GM-CSF facilitates DC generation, while IL-4 inhibits the differentiation of DC precursor cells into CD14+ macrophages, manipulating its differentiation into CD14+-CD1a+- DCs. However, the addition of other growth factors such as TNF-α, IFN-β, IFN-γ, IL-6, and PGE2 are also necessary to induce differentiation into mature CD1a+CD83+ DCs, which can be used for subsequent cell therapy production processes.
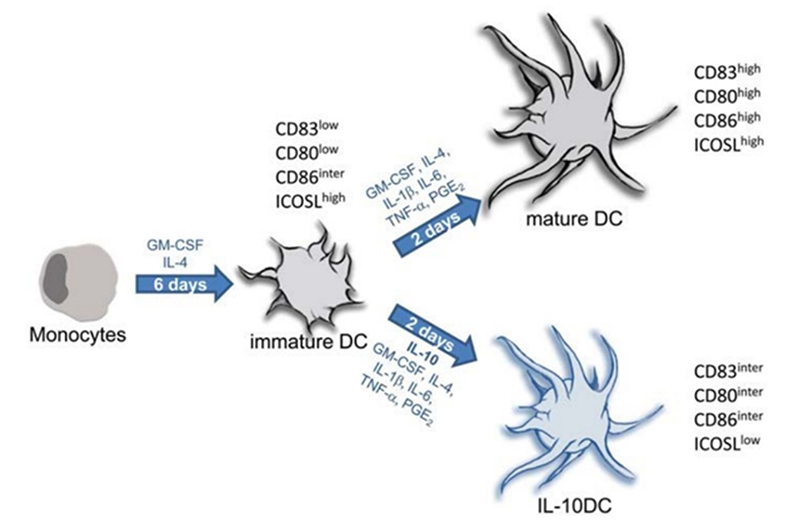
Protocols for generation of tolerogenic and immunogenic dendritic cells from monocytic precursors. Frontiers in immunology, 4, 82.
DC vaccines are revolutionizing cancer treatment by leveraging the body's immune system to target cancer cells. While Sipuleucel-T (Provenge) is the sole FDA-approved DC vaccine for metastatic castrate-resistant prostate cancer, other DC vaccines have gained approval worldwide for various cancer types. Examples include HybriCell in Brazil, CreaVax-RCC in Korea, and APCEDEN in India. Additionally, DCVax-L shows promise for glioblastoma treatment in Germany. These vaccines utilize a patient's own DCs, loaded with tumor antigens used to then provoke an immune response against cancer.
The landscape of DC vaccine research is expansive, with over 300 clinical trials exploring their potential across different malignancies. These trials investigate methods to enhance vaccine efficacy, such as utilizing cytokine cocktails and combinatorial treatments with chemotherapy or immune checkpoint inhibitors. This approach holds immense promise for improving cancer treatment outcomes, offering personalized and targeted vaccines tailored to individual patients.
DC stands as a versatile orchestrator capable of activating naive T cells, making them indispensable in the body's immune system. Over the past two decades, research on DC vaccines/cell therapy has made significant strides, enhancing the migratory capabilities and immune efficacy of DCs. However, our understanding of DC subsets, functions, disease mechanisms, and targets remains limited, requires deeper exploration of these foundational understanding for breakthroughs in DC vaccine development. Nevertheless, with an increasing number of DC vaccines undergoing clinical trials, it seems that DC cell immunotherapy continue to grow into an even bigger role in cancer treatment in the future.
This web search service is supported by Google Inc.









