 Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order. Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
 Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!  Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| CTB-M52H9 | Mouse | Mouse Cathepsin B / CTSB Protein, His Tag (active enzyme, MALS verified) |  |

|
|
| CTB-H5222 | Human | Human Cathepsin B / CTSB Protein, His Tag (active enzyme, MALS verified) | 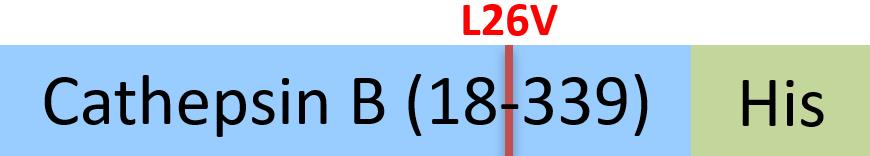 |

|
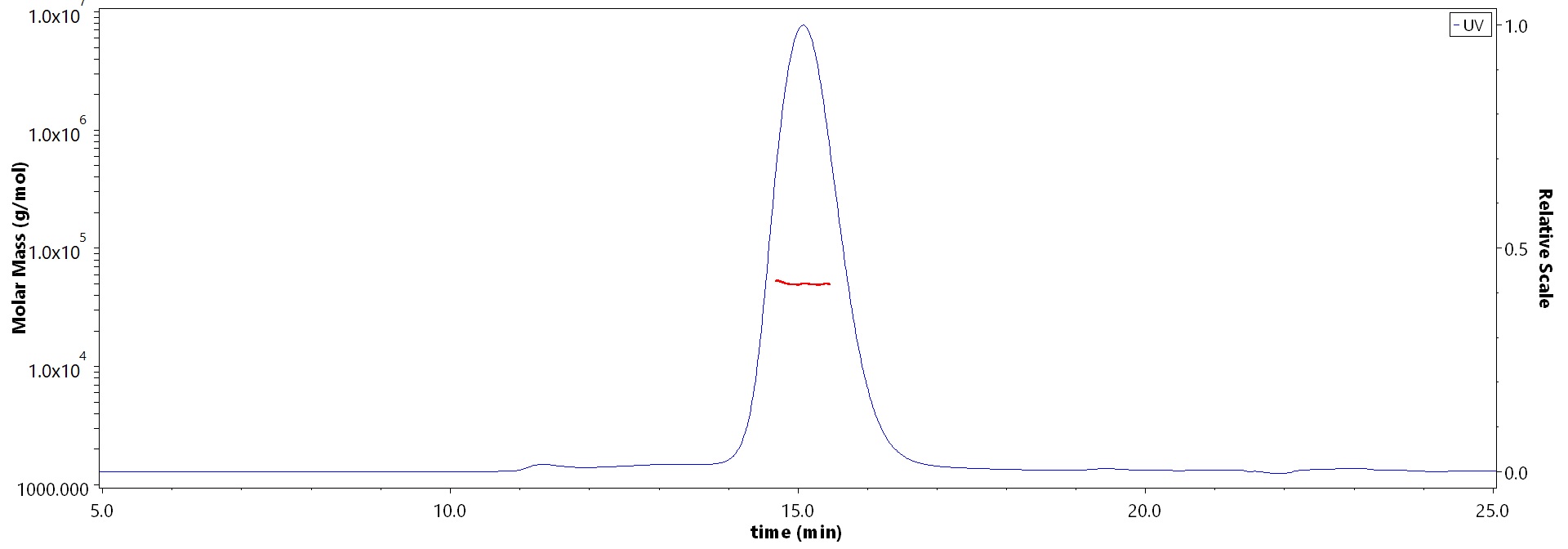
The purity of Mouse Cathepsin B, His Tag (Cat. No. CTB-M52H9) is more than 90% and the molecular weight of this protein is around 45-55 kDa verified by SEC-MALS.
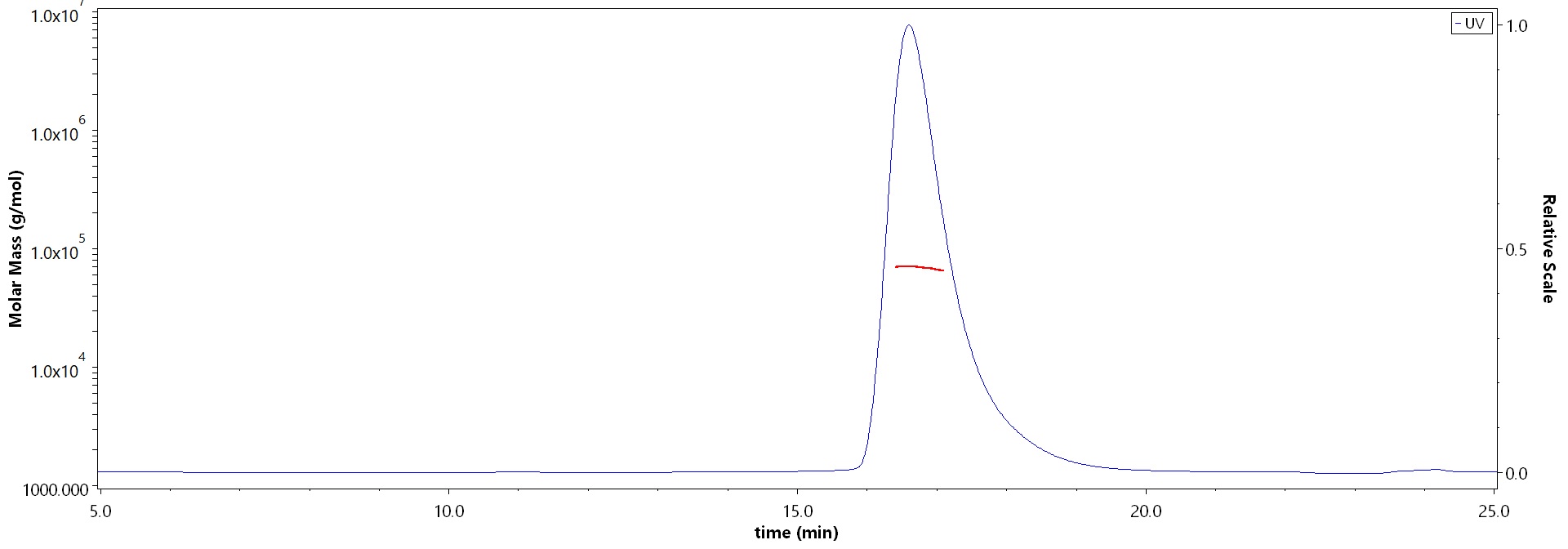
The purity of Human Cathepsin B, His Tag (Cat. No. CTB-H5222) is more than 90% and the molecular weight of this protein is around 60-75 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Osugacestat | AL-101(BMS); BMS-906024 | Phase 2 Clinical | Bristol-Myers Squibb Company, Glaxosmithkline Plc | Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Adenoid Cystic; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma | Details |
This web search service is supported by Google Inc.
